Assessment of Proton Beam Therapy Use Among Patients With Newly Diagnosed Cancer in the US, 2004-2018
- PMID: 35476066
- PMCID: PMC9047654
- DOI: 10.1001/jamanetworkopen.2022.9025
Assessment of Proton Beam Therapy Use Among Patients With Newly Diagnosed Cancer in the US, 2004-2018
Abstract
Importance: Proton beam therapy (PBT) is a potentially superior technology to photon radiotherapy for tumors with complex anatomy, those surrounded by sensitive tissues, and childhood cancers.
Objective: To assess patterns of use of PBT according to the present American Society of Radiation Oncology (ASTRO) clinical indications in the US.
Design, setting, and participants: Individuals newly diagnosed with cancer between 2004 and 2018 were selected from the National Cancer Database. Data analysis was performed from October 4, 2021, to February 22, 2022. ASTRO's Model Policies (2017) were used to classify patients into group 1, for which health insurance coverage for PBT treatment is recommended, and group 2, for which coverage is recommended only if additional requirements are met.
Main outcomes and measures: Use of PBT.
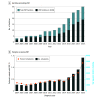
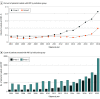
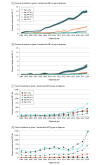
Results: Of the 5 919 368 patients eligible to receive PBT included in the study, 3 206 902 were female (54.2%), and mean (SD) age at diagnosis was 62.6 (12.3) years. Use of PBT in the US increased from 0.4% in 2004 to 1.2% in 2018 (annual percent change [APC], 8.12%; P < .001) due to increases in group 1 from 0.4% in 2010 to 2.2% in 2018 (APC, 21.97; P < .001) and increases in group 2 from 0.03% in 2014 to 0.1% in 2018 (APC, 30.57; P < .001). From 2010 to 2018, among patients in group 2, PBT targeted to the breast increased from 0.0% to 0.9% (APC, 51.95%), and PBT targeted to the lung increased from 0.1% to 0.7% (APC, 28.06%) (P < .001 for both). Use of PBT targeted to the prostate decreased from 1.4% in 2011 to 0.8% in 2014 (APC, -16.48%; P = .03) then increased to 1.3% in 2018 (APC, 12.45; P < .001). Most patients in group 1 treated with PBT had private insurance coverage in 2018 (1039 [55.4%]); Medicare was the most common insurance type among those in group 2 (1973 [52.5%]).
Conclusions and relevance: The findings of this study show an increase in the use of PBT in the US between 2004 to 2018; prostate was the only cancer site for which PBT use decreased temporarily between 2011 and 2014, increasing again between 2014 and 2018. These findings may be especially relevant for Medicare radiation oncology coverage policies.
Conflict of interest statement
Figures

References
-
- Brada M, Pijls-Johannesma M, De Ruysscher D. Current clinical evidence for proton therapy. Cancer J. 2009;15(4):319-324. - PubMed
-
- Jarosek S, Elliott S, Virnig B. Proton Beam Radiotherapy in the US Medicare Population: Growth in Use Between 2006 and 2009: Data Points # 10. Agency for Healthcare Research and Quality; 2012. - PubMed
-
- Hoppe BS, Flampouri S, Zaiden R, et al. . Involved-node proton therapy in combined modality therapy for Hodgkin lymphoma: results of a phase 2 study. Int J Radiat Oncol Biol Phys. 2014;89(5):1053-1059. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

