Lactate induces vascular permeability via disruption of VE-cadherin in endothelial cells during sepsis
- PMID: 35476437
- PMCID: PMC9045716
- DOI: 10.1126/sciadv.abm8965
Lactate induces vascular permeability via disruption of VE-cadherin in endothelial cells during sepsis
Abstract
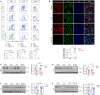
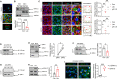
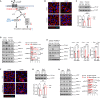
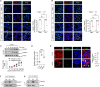
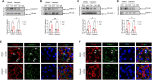
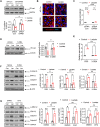
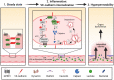
Circulating lactate levels are a critical biomarker for sepsis and are positively correlated with sepsis-associated mortality. We investigated whether lactate plays a biological role in causing endothelial barrier dysfunction in sepsis. We showed that lactate causes vascular permeability and worsens organ dysfunction in CLP sepsis. Mechanistically, lactate induces ERK-dependent activation of calpain1/2 for VE-cadherin proteolytic cleavage, leading to the enhanced endocytosis of VE-cadherin in endothelial cells. In addition, we found that ERK2 interacts with VE-cadherin and stabilizes VE-cadherin complex in resting endothelial cells. Lactate-induced ERK2 phosphorylation promotes ERK2 disassociation from VE-cadherin. In vivo suppression of lactate production or genetic depletion of lactate receptor GPR81 mitigates vascular permeability and multiple organ injury and improves survival outcome in polymicrobial sepsis. Our study reveals that metabolic cross-talk between glycolysis-derived lactate and the endothelium plays a critical role in the pathophysiology of sepsis.
Figures


References
-
- Singer M., Deutschman C. S., Seymour C. W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G. R., Chiche J. D., Coopersmith C. M., Hotchkiss R. S., Levy M. M., Marshall J. C., Martin G. S., Opal S. M., Rubenfeld G. D., van der Poll T., Vincent J. L., Angus D. C., The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315, 801–810 (2016). - PMC - PubMed
-
- Joffre J., Hellman J., Ince C., Ait-Oufella H., Endothelial responses in sepsis. Am. J. Respir. Crit. Care Med. 202, 361–370 (2020). - PubMed
-
- Goldenberg N. M., Steinberg B. E., Slutsky A. S., Lee W. L., Broken barriers: A new take on sepsis pathogenesis. Sci. Transl. Med. 3, 88ps25 (2011). - PubMed
-
- Jaffee W., Hodgins S., McGee W. T., Tissue edema, fluid balance, and patient outcomes in severe sepsis: An organ systems review. J. Intensive Care Med. 33, 502–509 (2018). - PubMed
-
- Lee W. L., Slutsky A. S., Sepsis and endothelial permeability. N. Engl. J. Med. 363, 689–691 (2010). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

