Distinct Neoadjuvant Chemotherapy Response and 5-Year Outcome in Patients With Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Breast Tumors That Reclassify as Basal-Type by the 80-Gene Signature
- PMID: 35476550
- PMCID: PMC9200401
- DOI: 10.1200/PO.21.00463
Distinct Neoadjuvant Chemotherapy Response and 5-Year Outcome in Patients With Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Breast Tumors That Reclassify as Basal-Type by the 80-Gene Signature
Abstract
Purpose: The 80-gene molecular subtyping signature (80-GS) reclassifies a proportion of immunohistochemistry (IHC)-defined luminal breast cancers (estrogen receptor-positive [ER+], human epidermal growth factor receptor 2-negative [HER2-]) as Basal-Type. We report the association of 80-GS reclassification with neoadjuvant treatment response and 5-year outcome in patients with breast cancer.
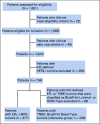
Methods: Neoadjuvant Breast Registry Symphony Trial (NBRST; NCT01479101) is an observational, prospective study that included 1,069 patients with early-stage breast cancer age 18-90 years who received neoadjuvant therapy. Pathologic complete response (pCR) and 5-year distant metastasis-free survival (DMFS) and overall survival (OS) were assessed in 477 patients with IHC-defined ER+, HER2- tumors and in a reference group of 229 patients with IHC-defined triple-negative breast cancer (TNBC).
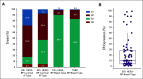
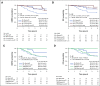
Results: 80-GS reclassified 15% of ER+, HER2- tumors (n = 73) as Basal-Type (ER+/Basal), which had similar pCR compared with TNBC/Basal tumors (34% v 38%; P = .52), and significantly higher pCR than ER+/Luminal A (2%; P < .001) and ER+/Luminal B (6%; P < .001) tumors. The 5-year DMFS (%, [95% CI]) was significantly lower for patients with ER+/Basal tumors (66% [52.6 to 77.3]), compared with those with ER+/Luminal A tumors (92.3% [85.2 to 96.1]) and ER+/Luminal B tumors (73.5% [44.5 to 79.3]). Importantly, patients with ER+/Basal or TNBC/Basal tumors that had a pCR exhibited significantly improved DMFS and OS compared with those with residual disease. By contrast, patients with ER+/Luminal B tumors had comparable 5-year DMFS and OS whether or not they achieved pCR.
Conclusion: Significant differences in chemosensitivity and 5-year outcome suggest patients with ER+/Basal molecular subtype may benefit from neoadjuvant regimens optimized for patients with TNBC/Basal tumors compared with patients with ER+/Luminal subtype. These data highlight the importance of identifying this subset of patients to improve treatment planning and long-term survival.
Conflict of interest statement
Figures

Comment in
-
Reclassfying Estrogen Receptor-Positive/Basal Subtype by Using Immunohistochemistry Itself According to Grade and Estrogen Receptor Positivity Might Be Possible.JCO Precis Oncol. 2022 Aug;6:e2200242. doi: 10.1200/PO.22.00242. JCO Precis Oncol. 2022. PMID: 35952317 No abstract available.
-
Reply to W. Altundag.JCO Precis Oncol. 2022 Aug;6:e2200315. doi: 10.1200/PO.22.00315. JCO Precis Oncol. 2022. PMID: 35952323 No abstract available.
References
-
- Weigelt B, Baehner FL, Reis-Filho JS: The contribution of gene expression profiling to breast cancer classification, prognostication and prediction: A retrospective of the last decade. J Pathol 220:263-280, 2010 - PubMed
-
- Perou CM, Sorlie T, Eisen MB, et al. : Molecular portraits of human breast tumours. Nature 406:747-752, 2000 - PubMed
-
- Krijgsman O, Roepman P, Zwart W, et al. : A diagnostic gene profile for molecular subtyping of breast cancer associated with treatment response. Breast Cancer Res Treat 133:37-47, 2012 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

