Dimerization of the pulmonary surfactant protein C in a membrane environment
- PMID: 35476695
- PMCID: PMC9045638
- DOI: 10.1371/journal.pone.0267155
Dimerization of the pulmonary surfactant protein C in a membrane environment
Abstract
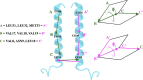
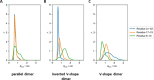
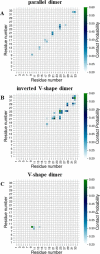
Surfactant protein C (SP-C) has several functions in pulmonary surfactant. These include the transfer of lipids between different membrane structures, a role in surfactant recycling and homeostasis, and involvement in modulation of the innate defense system. Despite these important functions, the structures of functional SP-C complexes have remained unclear. SP-C is known to exist as a primarily α-helical structure with an apparently unstructured N-terminal region, yet there is recent evidence that the functions of SP-C could be associated with the formation of SP-C dimers and higher oligomers. In this work, we used molecular dynamics simulations, two-dimensional umbrella sampling, and well-tempered metadynamics to study the details of SP-C dimerization. The results suggest that SP-C dimerizes in pulmonary surfactant membranes, forming dimers of different topologies. The simulations identified a dimerization motif region V21xxxVxxxGxxxM33 that is much larger than the putative A30xxxG34 motif that is commonly assumed to control the dimerization of some α-helical transmembrane domains. The results provide a stronger basis for elucidating how SP-C functions in concert with other surfactant proteins.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
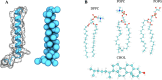




References
-
- Singh SK. Human respiratory viral infections: CRC Press; 2014.
-
- Perez-Gil J, Casals C, Marsh D. Interactions of hydrophobic lung surfactant proteins SP-B and SP-C with dipalmitoylphosphatidylcholine and dipalmitoylphosphatidylglycerol bilayers studied by electron spin resonance spectroscopy. Biochemistry. 1995;34(12):3964–71. doi: 10.1021/bi00012a014 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

