Botulinum Injection Into the Proximal Intestinal Wall of Diet-Induced Obese Mice Leads to Weight Loss and Improves Glucose and Fat Tolerance
- PMID: 35476783
- PMCID: PMC9490449
- DOI: 10.2337/db21-0708
Botulinum Injection Into the Proximal Intestinal Wall of Diet-Induced Obese Mice Leads to Weight Loss and Improves Glucose and Fat Tolerance
Abstract
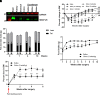
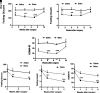
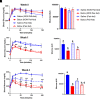
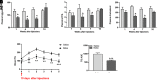
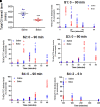
Botulinum neurotoxin (available commercially as BOTOX) has been used successfully for treatment of several neuromuscular disorders, including blepharospasm, dystonia, spasticity, and cerebral palsy in children. Our data demonstrate that injection of Botox into the proximal intestinal wall of diet-induced obese (DIO) mice induces weight loss and reduces food intake. This was associated with amelioration of hyperglycemia, hyperlipidemia, and significant improvement of glucose tolerance without alteration of energy expenditure. We also observed accelerated gastrointestinal transit and significant reductions in glucose and lipid absorption, which may account, at least in part, for the observed weight loss and robust metabolic benefits, although possible systemic effects occurring as a consequence of central and/or peripheral signaling cannot be ignored. The observed metabolic benefits were found to be largely independent of weight loss, as demonstrated by pair-feeding experiments. Effects lasted ∼8 weeks, for as long as the half-life of Botox as reported in prior rodent studies. These results have valuable clinical implications. If the observed effects are translatable in humans, this approach could lay the foundation for therapeutic approaches geared toward robust and sustained weight loss, mimicking some of the benefits of bariatric operations without its cost and complications.
© 2022 by the American Diabetes Association.
Figures



References
-
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults, United States, 2017–2018. NCHS Data Brief, no 360. Accessed 19 May 2022. Available from https://www.cdc.gov/nchs/data/databriefs/db360-h.pdf - PubMed
-
- Safaei M, Sundararajan EA, Driss M, Boulila W, Shapi’I A. A systematic literature review on obesity: understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput Biol Med 2021;136:104754. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

