Breakage of cytoplasmic chromosomes by pathological DNA base excision repair
- PMID: 35477155
- PMCID: PMC10680091
- DOI: 10.1038/s41586-022-04767-1
Breakage of cytoplasmic chromosomes by pathological DNA base excision repair
Abstract
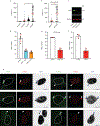
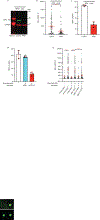
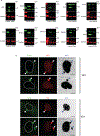
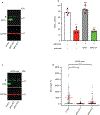
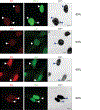
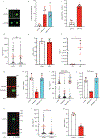
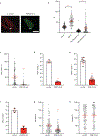
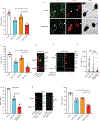
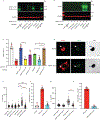
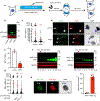
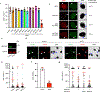
Chromothripsis is a catastrophic mutational process that promotes tumorigenesis and causes congenital disease1-4. Chromothripsis originates from aberrations of nuclei called micronuclei or chromosome bridges5-8. These structures are associated with fragile nuclear envelopes that spontaneously rupture9,10, leading to DNA damage when chromatin is exposed to the interphase cytoplasm. Here we identify a mechanism explaining a major fraction of this DNA damage. Micronuclei accumulate large amounts of RNA-DNA hybrids, which are edited by adenine deaminases acting on RNA (ADAR enzymes) to generate deoxyinosine. Deoxyinosine is then converted into abasic sites by a DNA base excision repair (BER) glycosylase, N-methyl-purine DNA glycosylase11,12 (MPG). These abasic sites are cleaved by the BER endonuclease, apurinic/apyrimidinic endonuclease12 (APE1), creating single-stranded DNA nicks that can be converted to DNA double strand breaks by DNA replication or when closely spaced nicks occur on opposite strands13,14. This model predicts that MPG should be able to remove the deoxyinosine base from the DNA strand of RNA-DNA hybrids, which we demonstrate using purified proteins and oligonucleotide substrates. These findings identify a mechanism for fragmentation of micronuclear chromosomes, an important step in generating chromothripsis. Rather than breaking any normal chromosome, we propose that the eukaryotic cytoplasm only damages chromosomes with pre-existing defects such as the DNA base abnormality described here.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interest
D. Pellman is a member of the Volastra Therapeutics SAB.
Figures



Comment in
-
A shattering experience.Mol Cell. 2022 Jul 7;82(13):2360-2362. doi: 10.1016/j.molcel.2022.06.018. Mol Cell. 2022. PMID: 35803217
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

