Targeting nucleotide metabolism: a promising approach to enhance cancer immunotherapy
- PMID: 35477416
- PMCID: PMC9044757
- DOI: 10.1186/s13045-022-01263-x
Targeting nucleotide metabolism: a promising approach to enhance cancer immunotherapy
Abstract
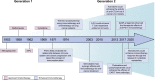
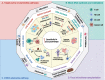
Targeting nucleotide metabolism can not only inhibit tumor initiation and progression but also exert serious side effects. With in-depth studies of nucleotide metabolism, our understanding of nucleotide metabolism in tumors has revealed their non-proliferative effects on immune escape, indicating the potential effectiveness of nucleotide antimetabolites for enhancing immunotherapy. A growing body of evidence now supports the concept that targeting nucleotide metabolism can increase the antitumor immune response by (1) activating host immune systems via maintaining the concentrations of several important metabolites, such as adenosine and ATP, (2) promoting immunogenicity caused by increased mutability and genomic instability by disrupting the purine and pyrimidine pool, and (3) releasing nucleoside analogs via microbes to regulate immunity. Therapeutic approaches targeting nucleotide metabolism combined with immunotherapy have achieved exciting success in preclinical animal models. Here, we review how dysregulated nucleotide metabolism can promote tumor growth and interact with the host immune system, and we provide future insights into targeting nucleotide metabolism for immunotherapeutic treatment of various malignancies.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Weaver BA, Cleveland DW. Decoding the links between mitosis, cancer, and chemotherapy: the mitotic checkpoint, adaptation, and cell death. Cancer Cell. 2005;8(1):7–12. - PubMed
-
- Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. N Engl J Med. 2001;344(26):1997–2008. - PubMed
-
- Schein PS, Winokur SH. Immunosuppressive and cytotoxic chemotherapy: long-term complications. Ann Intern Med. 1975;82(1):84–95. - PubMed
-
- Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369(6510):1481–1489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

