Pharmacological activation of ATF6 remodels the proteostasis network to rescue pathogenic GABAA receptors
- PMID: 35477478
- PMCID: PMC9044816
- DOI: 10.1186/s13578-022-00783-w
Pharmacological activation of ATF6 remodels the proteostasis network to rescue pathogenic GABAA receptors
Abstract
Background: Genetic variants in the subunits of the gamma-aminobutyric acid type A (GABAA) receptors are implicated in the onset of multiple pathologic conditions including genetic epilepsy. Previous work showed that pathogenic GABAA subunits promote misfolding and inefficient assembly of the GABAA receptors, limiting receptor expression and activity at the plasma membrane. However, GABAA receptors containing variant subunits can retain activity, indicating that enhancing the folding, assembly, and trafficking of these variant receptors offers a potential opportunity to mitigate pathology associated with genetic epilepsy.
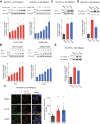
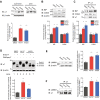
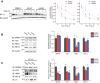
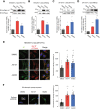
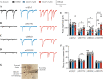
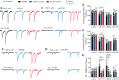
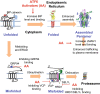
Results: Here, we demonstrate that pharmacologically enhancing endoplasmic reticulum (ER) proteostasis using small molecule activators of the ATF6 (Activating Transcription Factor 6) signaling arm of the unfolded protein response (UPR) increases the assembly, trafficking, and surface expression of variant GABAA receptors. These improvements are attributed to ATF6-dependent remodeling of the ER proteostasis environment, which increases protein levels of pro-folding ER proteostasis factors including the ER chaperone BiP (Immunoglobulin Binding Protein) and trafficking receptors, such as LMAN1 (Lectin Mannose-Binding 1) and enhances their interactions with GABAA receptors. Importantly, we further show that pharmacologic ATF6 activators increase the activity of GABAA receptors at the cell surface, revealing the potential for this strategy to restore receptor activity to levels that could mitigate disease pathogenesis.
Conclusions: These results indicate that pharmacologic ATF6 activators offer an opportunity to restore GABAA receptor activity in diseases including genetic epilepsy and point to the potential for similar pharmacologic enhancement of ER proteostasis to improve trafficking of other disease-associated variant ion channels implicated in etiologically-diverse diseases.
© 2022. The Author(s).
Conflict of interest statement
RLW and JWK are inventors on patents describing ATF6 activator compounds, including AA147 and AA263. RLW and JWK are also shareholders and scientific advisory board members of Protego Biopharma who have licensed the ATF6 activators AA147 and AA263.
Figures
References
-
- X. Fu, Y. J. Wang, J. Q. Kang and T. W. Mu GABAA Receptor Variants in Epilepsy, In: Czuczwar SJ, editor. Epilepsy. Brisbane (AU): Exon Publications. Doi: 10.36255/exon-publications-epilepsy-gaba-receptor - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

