High altitude is associated with pTau deposition, neuroinflammation, and myelin loss
- PMID: 35477957
- PMCID: PMC9046305
- DOI: 10.1038/s41598-022-10881-x
High altitude is associated with pTau deposition, neuroinflammation, and myelin loss
Abstract
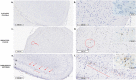
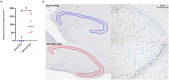
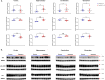
Mammals are able to adapt to high altitude (HA) if appropriate acclimation occurs. However, specific occupations (professional climbers, pilots, astronauts and other) can be exposed to HA without acclimation and be at a higher risk of brain consequences. In particular, US Air Force U2-pilots have been shown to develop white matter hyperintensities (WMH) on MRI. Whether WMH are due to hypoxia or hypobaria effects is not understood. We compared swine brains exposed to 5000 feet (1524 m) above sea level (SL) with 21% fraction inspired O2 (FiO2) (Control group [C]; n = 5) vs. 30,000 feet (9144 m) above SL with 100% FiO2 group (hypobaric group [HYPOBAR]; n = 6). We performed neuropathologic assessments, molecular analyses, immunohistochemistry (IHC), Western Blotting (WB), and stereology analyses to detect differences between HYPOBAR vs. Controls. Increased neuronal insoluble hyperphosphorylated-Tau (pTau) accumulation was observed across different brain regions, at histological level, in the HYPOBAR vs. Controls. Stereology-based cell counting demonstrated a significant difference (p < 0.01) in pTau positive neurons between HYPOBAR and C in the Hippocampus. Higher levels of soluble pTau in the Hippocampus of HYPOBAR vs. Controls were also detected by WB analyses. Additionally, WB demonstrated an increase of IBA-1 in the Cerebellum and a decrease of myelin basic protein (MBP) in the Hippocampus and Cerebellum of HYPOBAR vs. Controls. These findings illustrate, for the first time, changes occurring in large mammalian brains after exposure to nonhypoxic-hypobaria and open new pathophysiological views on the interaction among hypobaria, pTau accumulation, neuroinflammation, and myelination in large mammals exposed to HA.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

