Radiomitigation by Melatonin in C57BL/6 Mice: Possible Implications as Adjuvant in Radiotherapy and Chemotherapy
- PMID: 35478105
- PMCID: PMC9087054
- DOI: 10.21873/invivo.12820
Radiomitigation by Melatonin in C57BL/6 Mice: Possible Implications as Adjuvant in Radiotherapy and Chemotherapy
Abstract
Background/aim: Melatonin (N-acetyl-5-methoxytryptamine), a chief secretory molecule of the pineal gland, has multiple properties, and numerous clinical investigations regarding its actions are in progress. This study investigated the radiomitigative role of melatonin in C57BL/6 mice.
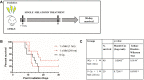
Materials and methods: Melatonin (100 mg/kg) was orally administered once daily starting at 1 h on day 1 and subsequently every 24 h until day 7 after whole-body irradiation (WBI) and survival was monitored for 30 days. The bone marrow, spleen, and intestine were studied to evaluate the mitigative potential of melatonin after radiation-induced damage.
Results: Melatonin significantly improved the survival upto 60% and 90% after 9 Gy (lethal) and 7.5 Gy (sub-lethal) WBI, respectively. Melatonin alleviated WBI-induced myelosuppression and pancytopenia, and increased white blood cell, red blood cell, platelet, and lymphocyte (CD4+ and CD8+) counts in peripheral blood. Bone marrow and spleen cellularity were restored through enhanced haematopoiesis. Melatonin ameliorated the damage in the small intestine, and promoted recovery of villi length, crypts number, and goblet cell count.
Conclusion: Melatonin mitigates the radiation-induced injury in the gastrointestinal and haematopoietic systems. The observed radiomitigative properties of melatonin can also be useful in the context of adjuvant therapy for cancer and radiotherapy.
Keywords: Melatonin; bone marrow; cancer treatment; gastrointestinal; haematopoietic; radiomitigator.
Copyright © 2022, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors report no conflicts of interest regarding this study. The Authors alone are responsible for the content and writing of the paper.
Figures









Similar articles
-
Melatonin attenuates 60 Co γ-ray-induced hematopoietic, immunological and gastrointestinal injuries in C57BL/6 male mice.Environ Toxicol. 2017 Feb;32(2):501-518. doi: 10.1002/tox.22254. Epub 2016 Mar 7. Environ Toxicol. 2017. PMID: 26948951
-
Sesamol attenuates genotoxicity in bone marrow cells of whole-body γ-irradiated mice.Mutagenesis. 2015 Sep;30(5):651-61. doi: 10.1093/mutage/gev026. Epub 2015 Apr 11. Mutagenesis. 2015. PMID: 25863274
-
Mitigation of Radiation-Induced Gastrointestinal System Injury by Melatonin: A Histopathological Study.Curr Drug Res Rev. 2020 Jun 20;12(1):72-79. doi: 10.2174/2589977511666191031094625. Curr Drug Res Rev. 2020. PMID: 32578524
-
Protection of rat chromosomes by melatonin against gamma radiation-induced damage.Mutat Res. 2009 Jun-Jul;677(1-2):14-20. doi: 10.1016/j.mrgentox.2009.04.016. Epub 2009 May 22. Mutat Res. 2009. PMID: 19465147
-
A radiobiological review on melatonin: a novel radioprotector.J Radiat Res. 2007 Jul;48(4):263-72. doi: 10.1269/jrr.06070. J Radiat Res. 2007. PMID: 17641465 Review.
Cited by
-
A genetic investigation in five Chinese families with keratoconus.PeerJ. 2024 Sep 2;12:e18037. doi: 10.7717/peerj.18037. eCollection 2024. PeerJ. 2024. PMID: 39238827 Free PMC article.
-
Potential of melatonin to reverse epigenetic aberrations in oral cancer: new findings.EXCLI J. 2023 Dec 12;22:1280-1310. doi: 10.17179/excli2023-6624. eCollection 2023. EXCLI J. 2023. PMID: 38234969 Free PMC article. Review.
-
Synthetic and Natural Radioprotective Agents: Recent Status and their Underlying Mechanism of Action.Curr Pharm Biotechnol. 2025;26(5):700-715. doi: 10.2174/0113892010293722240522071042. Curr Pharm Biotechnol. 2025. PMID: 38818911 Review.
-
The effect of myeloablative radiation on urinary bladder mast cells.Sci Rep. 2024 Mar 14;14(1):6219. doi: 10.1038/s41598-024-56655-5. Sci Rep. 2024. PMID: 38485999 Free PMC article.
-
Melatonin Type 2 Receptor Activation Regulates Blue Light Exposure-Induced Mouse Corneal Epithelial Damage by Modulating Impaired Autophagy and Apoptosis.Int J Mol Sci. 2022 Sep 26;23(19):11341. doi: 10.3390/ijms231911341. Int J Mol Sci. 2022. PMID: 36232639 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
