Anti-tuberculosis treatment strategies and drug development: challenges and priorities
- PMID: 35478222
- PMCID: PMC9045034
- DOI: 10.1038/s41579-022-00731-y
Anti-tuberculosis treatment strategies and drug development: challenges and priorities
Abstract
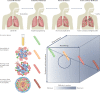
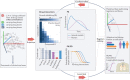
Despite two decades of intensified research to understand and cure tuberculosis disease, biological uncertainties remain and hamper progress. However, owing to collaborative initiatives including academia, the pharmaceutical industry and non-for-profit organizations, the drug candidate pipeline is promising. This exceptional success comes with the inherent challenge of prioritizing multidrug regimens for clinical trials and revamping trial designs to accelerate regimen development and capitalize on drug discovery breakthroughs. Most wanted are markers of progression from latent infection to active pulmonary disease, markers of drug response and predictors of relapse, in vitro tools to uncover synergies that translate clinically and animal models to reliably assess the treatment shortening potential of new regimens. In this Review, we highlight the benefits and challenges of 'one-size-fits-all' regimens and treatment duration versus individualized therapy based on disease severity and host and pathogen characteristics, considering scientific and operational perspectives.
© 2022. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- World Health Organization. Global tuberculosis report 2020 (WHO, 2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

