Effects of dapagliflozin on volume status and systemic haemodynamics in patients with chronic kidney disease without diabetes: Results from DAPASALT and DIAMOND
- PMID: 35478433
- PMCID: PMC9262818
- DOI: 10.1111/dom.14729
Effects of dapagliflozin on volume status and systemic haemodynamics in patients with chronic kidney disease without diabetes: Results from DAPASALT and DIAMOND
Abstract
Aims: To assess the effect of sodium-glucose cotransporter-2 inhibitor dapagliflozin on natriuresis, blood pressure (BP) and volume status in patients with chronic kidney disease (CKD) without diabetes.
Materials and methods: We performed a mechanistic open-label study (DAPASALT) to evaluate the effects of dapagliflozin on 24-hour sodium excretion, 24-hour BP, extracellular volume, and markers of volume status during a standardized sodium diet (150 mmol/d) in six patients with CKD. In parallel, in a placebo-controlled double-blind crossover trial (DIAMOND), we determined the effects of 6 weeks of dapagliflozin on markers of volume status in 53 patients with CKD.
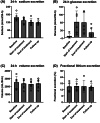
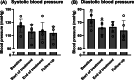
Results: In DAPASALT (mean age 65 years, mean estimated glomerular filtration rate [eGFR] 39.4 mL/min/1.73 m2 , median urine albumin:creatinine ratio [UACR] 111 mg/g), dapagliflozin did not change 24-hour sodium and volume excretion during 2 weeks of treatment. Dapagliflozin was associated with a modest increase in 24-hour glucose excretion on Day 4, which persisted at Day 14 and reversed to baseline after discontinuation. Mean 24-hour systolic BP decreased by -9.3 (95% confidence interval [CI] -19.1, 0.4) mmHg after 4 days and was sustained at Day 14 and at wash-out. Renin, angiotensin II, urinary aldosterone and copeptin levels increased from baseline. In DIAMOND (mean age 51 years, mean eGFR 59.0 mL/min/1.73 m2 , median UACR 608 mg/g), compared to placebo, dapagliflozin increased plasma renin (38.5 [95% CI 7.4, 78.8]%), aldosterone (19.1 [95% CI -5.9, 50.8]%), and copeptin levels (7.3 [95% CI 0.1, 14.5] pmol/L).
Conclusions: During a standardized sodium diet, dapagliflozin decreased BP but did not increase 24-hour sodium and volume excretion. The lack of increased natriuresis and diuresis may be attributed to activation of intra-renal compensatory mechanisms to prevent excessive water loss.
Keywords: SGLT2 inhibitor; adaptive response; dapagliflozin; kidney.
© 2022 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
T. Sen, R. Scholtes, C.C.J. Dekkers, Q. Li, S. Barbour and A.H.J. Danser have nothing to disclose. P.J Greasley, C. Karlsson and A. Hammarstedt are AstraZeneca employees and shareholders. D.Z.I.C has received honoraria from Boehringer Ingelheim‐Lilly, Merck, AstraZeneca, Sanofi, Mitsubishi‐Tanabe, Abbvie, Janssen, Bayer, Prometic, BMS, Maze, CSL‐Behring, Otsuka, Novartis and Novo‐Nordisk, and has received operational funding for clinical trials from Boehringer Ingelheim‐Lilly, Merck, Janssen, Sanofi, AstraZeneca and Novo‐Nordisk. M. Vervloet has received consulting fees from Amgen, Vifor Fresenius Medical Care Renal Pharma, Medice, Cablon Medical, Otsuka and Kyowa Kirin. G.D. Laverman has served on advisory boards of Boehringer Ingelheim, Eli Lilly Alliance, Sanofi, Novo Nordisk, AstraZeneca and Vifor Pharma, and received research grants from AstraZeneca, Sanofi, Novo Nordisk, Vifor Pharma. P. Bjornstad. has acted as a consultant for AstraZeneca, Bayer, Bristol‐Myers Squibb, Boehringer Ingelheim, Eli‐Lilly, LG Chem, Sanofi, Novo Nordisk and Horizon Pharma. P.B. serves on the advisory boards for AstraZeneca, Bayer, Boehringer Ingelheim, Novo Nordisk and XORTX. D.H. van Raalte serves on advisory boards of Boehringer Ingelheim, Eli Lilly Alliance, Sanofi, Merck Sharp & Dohme (MSD) and Bayer, and received research grants from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Sanofi and MSD. H.J.L. Heerspink has served as a consultant for AbbVie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Pharma, Fresenius, Gilead, Janssen, Merck, Mundipharma, Mitsubishi Tanabe and Retrophin, and has received grant support from AbbVie, AstraZeneca, Boehringer Ingelheim and Janssen.
Figures

References
-
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295‐2306. - PubMed
-
- Heerspink HJL, Stefansson BV, Correa‐Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436‐1446. - PubMed
-
- Wheeler DC, Stefansson BV, Jongs N, et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non‐diabetic chronic kidney disease: a prespecified analysis from the DAPA‐CKD trial. Lancet Diabetes Endocrinol. 2021;9(1):22‐31. - PubMed
-
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995‐2008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

