A public-private collaboration model for clinical innovation
- PMID: 35478436
- PMCID: PMC9283745
- DOI: 10.1111/cts.13293
A public-private collaboration model for clinical innovation
Abstract
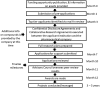
Launched in May 2012 as part of the New Therapeutic Uses program, the National Center for Advancing Translational Sciences (NCATS)' National Institutes of Health (NIH)-Industry Partnerships initiative fostered collaboration between pharmaceutical companies and the biomedical research community to advance therapeutic development. Over the 10-year life of the initiative, the industry partners included: AstraZeneca; AbbVie (formerly Abbott); Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; Janssen Pharmaceutical Research & Development, L.L.C.; Pfizer; Sanofi; and Mereo (out licensed assets). The initiative provided researchers at academic medical centers with a rare opportunity to propose clinical trials to test ideas for new therapeutic uses for a selection of clinic-ready and often previously proprietary experimental pharmaceutical assets that were provided by industry partners. Here, we describe the process by which collaborations between pharmaceutical companies with viable experimental assets and academic researchers with ideas for new uses of those assets were established; and how NCATS/NIH funding supported not only phase I and II clinical trials as well as any nonclinical studies needed before testing in a new patient population, it also provided an opportunity for testing innovative outcome measures for proof-of-concept trials. Although the program did not demonstrate improved success rates for phase II clinical trials, this collaboration model leverages the strengths of each party and with a focus toward evaluating an innovative outcome measure, could be used to reduce patient burden and trial costs, and improve patient engagement.
© 2022 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures


References
-
- DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ. 2016;47:20‐33. - PubMed
-
- Scannell JW, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov. 2012;11(3):191‐200. - PubMed
-
- Cook D, Brown D, Alexander R, et al. Lessons learned from the fate of AstraZeneca's drug pipeline: a five‐dimensional framework. Nat Rev Drug Discov. 2014;13(6):419‐431. - PubMed
-
- NIH , NIH RePort , in Reporter 2022. https://reporter.nih.gov/search/B9Xu1HDwMUKpl1maeyaAPg/projects?
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

