Hydroxysulfonylation of alkenes: an update
- PMID: 35478812
- PMCID: PMC9034158
- DOI: 10.1039/d1ra00513h
Hydroxysulfonylation of alkenes: an update
Abstract
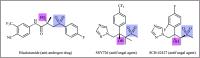
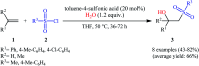
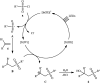
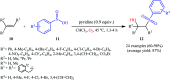
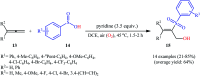
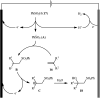
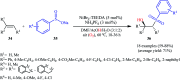
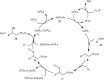
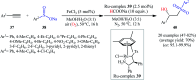
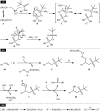
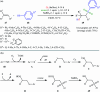
The direct difunctionalization of inexpensive and widely available alkenes has been recognized as a strong and straightforward tool for the rapid fabrication of complex molecules and pharmaceutical targets by introducing two different functional groups on adjacent carbon atoms of common alkene moieties in a single operation. This synthetic strategy avoids the purification and isolation of the intermediates and thus makes synthetic schemes shorter, simpler and cleaner. In this family of reactions, the hydroxysulfonylation of alkenes has emerged as an increasingly promising strategy for the synthesis of β-hydroxysulfones, which are found in many biologically important molecules and widespread applications in organic synthesis. The objective of this review is to illustrate the advancements in the field of hydroxysulfonylation of alkenes with special emphasis on the mechanistic details of the reaction pathways.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

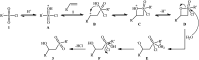


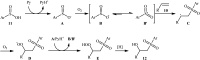


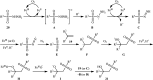


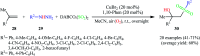


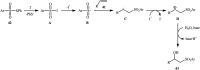






Similar articles
-
Palladium(II)-Catalyzed Oxidative Difunctionalization of Alkenes: Bond Forming at a High-Valent Palladium Center.Acc Chem Res. 2016 Nov 15;49(11):2413-2423. doi: 10.1021/acs.accounts.6b00328. Epub 2016 Oct 14. Acc Chem Res. 2016. PMID: 27739689
-
Intermolecular difunctionalization of alkenes: synthesis of β-hydroxy sulfides.RSC Adv. 2021 Apr 7;11(22):13138-13151. doi: 10.1039/d0ra09848e. eCollection 2021 Apr 7. RSC Adv. 2021. PMID: 35423843 Free PMC article. Review.
-
Aerobic Nickel-Catalyzed Hydroxysulfonylation of Alkenes Using Sodium Sulfinates.J Org Chem. 2015 Aug 7;80(15):7797-802. doi: 10.1021/acs.joc.5b01176. Epub 2015 Jul 17. J Org Chem. 2015. PMID: 26154403
-
Dioxygen concentration-dependent selective hydroxysulfonylation of olefins by rose bengal-sensitized photocatalysis.Org Biomol Chem. 2023 Oct 11;21(39):7994-8002. doi: 10.1039/d3ob01162c. Org Biomol Chem. 2023. PMID: 37755316
-
Difunctionalization of Alkenes Involving Metal Migration.Angew Chem Int Ed Engl. 2020 May 18;59(21):7990-8003. doi: 10.1002/anie.201913382. Epub 2020 Feb 25. Angew Chem Int Ed Engl. 2020. PMID: 31800977 Review.
Cited by
-
Direct vicinal sulfonyloximation of alkenes: an efficient and straightforward approach towards the synthesis of α-sulfonyl ketoximes.RSC Adv. 2025 May 22;15(22):17174-17185. doi: 10.1039/d5ra02712h. eCollection 2025 May 21. RSC Adv. 2025. PMID: 40406010 Free PMC article. Review.
-
Alkoxysulfenylation of alkenes: development and recent advances.RSC Adv. 2021 Oct 1;11(51):32513-32525. doi: 10.1039/d1ra03980f. eCollection 2021 Sep 27. RSC Adv. 2021. PMID: 35495514 Free PMC article. Review.
-
Direct selenosulfonylation of unsaturated compounds: a review.RSC Adv. 2022 Oct 26;12(47):30564-30576. doi: 10.1039/d2ra04128f. eCollection 2022 Oct 24. RSC Adv. 2022. PMID: 36337948 Free PMC article. Review.
References
-
- Cockshott I. D. Clin. Pharmacokinet. 2004;43:855–878. doi: 10.2165/00003088-200443130-00003. - DOI - PubMed
- Loebenberg D. Cacciapuoti A. Parmegiani R. Moss E. Menzel F. Antonacci B. Norris C. Yarosh-Tomaine T. Hare R. Miller G. Antimicrob. Agents Chemother. 1992;36:498–501. doi: 10.1128/AAC.36.2.498. - DOI - PMC - PubMed
- Walsh T. Lee J. Lecciones J. Kelly P. Peter J. Thomas V. Bacher J. Pizzo P. Antimicrob. Agents Chemother. 1990;34:1560–1564. doi: 10.1128/AAC.34.8.1560. - DOI - PMC - PubMed
-
- Mandai T. Yanagi T. Araki K. Morisaki Y. Kawada M. Otera J. J. Am. Chem. Soc. 1984;106:3670–3672. doi: 10.1021/ja00324a044. - DOI
- Solladie G. Frechou C. Demailly G. Greck C. J. Org. Chem. 1986;51:1912–1914. doi: 10.1021/jo00360a053. - DOI
- Tanikaga R. Hosoya K. Hamamura K. Kaji A. Tetrahedron Lett. 1987;28:3705–3706. doi: 10.1016/S0040-4039(00)96361-5. - DOI
- Robin S. Huet F. Fauve A. Veschambre H. Tetrahedron: Asymmetry. 1993;4:239–246. doi: 10.1016/S0957-4166(00)82344-7. - DOI
- Chang M.-Y. Lu Y.-J. Cheng Y.-C. Tetrahedron. 2015;71:1192–1201. doi: 10.1016/j.tet.2015.01.016. - DOI
-
- Bernabeu M. C. Bonete P. Caturla F. Chinchilla R. Nájera C. Tetrahedron: Asymmetry. 1996;7:2475–2478. doi: 10.1016/0957-4166(96)00310-2. - DOI
- Bertus P. Phansavath P. Ratovelomanana-Vidal V. Genêt J.-P. Touati A. Homri T. Hassine B. B. Tetrahedron Lett. 1999;40:3175–3178. doi: 10.1016/S0040-4039(99)00453-0. - DOI
Publication types
LinkOut - more resources
Full Text Sources

