Metal phthalocyanines: thin-film formation, microstructure, and physical properties
- PMID: 35478816
- PMCID: PMC9034105
- DOI: 10.1039/d1ra03853b
Metal phthalocyanines: thin-film formation, microstructure, and physical properties
Abstract
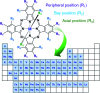
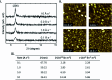
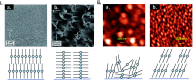
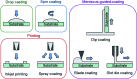
Metal phthalocyanines (MPcs) are an abundant class of small molecules comprising of a highly conjugated cyclic structure with a central chelated metal ion. Due to their remarkable chemical, mechanical, and thermal stability MPcs have become popular for a multitude of applications since their discovery in 1907. The potential for peripheral and axial functionalization affords structural tailoring to create bespoke MPc complexes for various next generation applications. Specifically, thin-films of MPcs have found promising utility in medical and electronic applications where the need to understand the relationship between chemical structure and the resulting thin-film properties is an important ongoing field. This review aims to compile the fundamental principles of small molecule thin-film formation by physical vapour deposition and solution processing focusing on the nucleation and growth of crystallites, thermodynamic and kinetic considerations, and effects of deposition parameters on MPc thin-films. Additionally, the structure-property relationship of MPc thin-films is examined by film microstructure, morphology and physical properties. The topics discussed in this work will elucidate the foundations of MPc thin-films and emphasize the critical need for not only molecular design of new MPcs but the role of their processing in the formation of thin-films and how this ultimately governs the performance of the resulting application.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




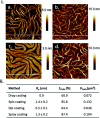





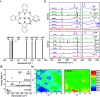

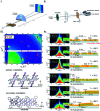


References
-
- Thomas A. L., Phthalocyanine Research and Applications, CRC Press, 1990
-
- McKeown N. B., Phthalocyanine Materials: Synthesis, Structure and Function, Cambridge University Press, 1998
-
- Moser T., The Phthalocyanines: Manufacture and Applications, CRC Press, 1983
-
- Newton M. I. Starke T. K. H. Willis M. R. McHale G. Sens. Actuators, B. 2000;67:307–311. doi: 10.1016/S0925-4005(00)00542-6. - DOI
Publication types
LinkOut - more resources
Full Text Sources

