Nano-porous Al/Au skeleton to support MnO2 with enhanced performance and electrodeposition adhesion for flexible supercapacitors
- PMID: 35478838
- PMCID: PMC9034163
- DOI: 10.1039/d1ra01923f
Nano-porous Al/Au skeleton to support MnO2 with enhanced performance and electrodeposition adhesion for flexible supercapacitors
Abstract
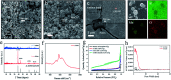
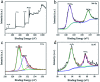
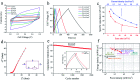
A nano-porous Al/Au skeleton is constructed to effectively improve the utilization rate of the active MnO2 and the overall adhesion between the current collector and MnO2 in an electrodeposition system. The Al/Au current collector is prepared by first forming a nano-porous structure on the surface of Al foil through etching modification, and subsequently coating an ultra-thin Au layer onto the Al foil. The active MnO2 is electrodeposited on the Al/Au current collector to fabricate a novel Al/Au/MnO2 electrode. The nano-porous skeleton supports MnO2 to grow autonomously inside-out. The ultra-thin Au layer acts as a transition layer to improve the overall conductivity of the current collector (0.35 Ω m-1) and to improve the adhesion with MnO2 as well. Owing to the highly porous structure, the electrochemical properties of the electrode are greatly improved, as evidenced by a remarkable specific capacitance of 222.13 mF cm-2 at 0.2 mA cm-2 and excellent rate capability of 63% capacitance retention at 6.0 mA cm-2. Furthermore, the assembled solid-state symmetric supercapacitor exhibits a high energy density of 0.68 mW h cm-3, excellent cyclic stability (86.3% capacitance retention after 2000 cycles), and prominent flexibility.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Burke A. J. Power Sources. 2000;91:37–50. doi: 10.1016/S0378-7753(00)00485-7. - DOI
LinkOut - more resources
Full Text Sources

