Analagesic and Anti-Inflammatory Potentials of a Less Ulcerogenic Thiadiazinethione Derivative in Animal Models: Biochemical and Histochemical Correlates
- PMID: 35478935
- PMCID: PMC9037714
- DOI: 10.2147/DDDT.S354779
Analagesic and Anti-Inflammatory Potentials of a Less Ulcerogenic Thiadiazinethione Derivative in Animal Models: Biochemical and Histochemical Correlates
Abstract
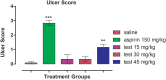
Purpose: Gastric ulcer induced by NSAIDs is the major medical concern and researchers are utilizing several approaches to combat this medical issue. In the current study, we investigated the efficacy of thiadiazinethione derivative (2,2'(2-thioxo-1,3,5-thiadiazinane-3,5-diyl) diacetic acid, as new less ulcerogenic compound.
Methods: 2,2'(2-thioxo-1,3,5-thiadiazinane-3,5-diyl) diacetic acid was evaluated using standard animal models including hot plate, writhing test and formalin induced nociceptive models. Anti-inflammatory activity was assessed via carrageenan-induced paw oedema model. Involvement of opioidergic nociceptive mechanism was confirmed via naloxone administration in hot plat assay. The gastro-ulcerogenic potential of test and standard compounds were evaluated via NSAID-induced pyloric ligation model followed by standard histopathological and biochemical analysis.
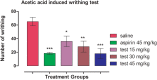
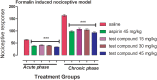
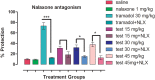
Results: In acetic acid-induced writhing test, our compound significantly reduced abdominal constrictions at the tested doses of 15 (p < 0.05), 30 (p < 0.01) and 45 mg kg-1 (p < 0.001) as compared to control (p < 0.001). In hot plate test, after 30 min of administration, our test compound showed significant anti-nociceptive potential (p < 0.05 at 15 and 30 mg kg-1 and p < 0.01 at 45 mg kg-1) and tramadol (p ˂ 0.001) at 30 mg kg-1 dose. After 60 min tramadol (30 kg-1) and test sample (30, 45 mg kg-1) exhibited significant anti-nociceptive activity p < 0.001. In Formalin-induced nociceptive response, a significant decline (p ˂ 0.001) was observed for aspirin and test compound during acute and chronic phases. Decline in the anti-nociceptive potential of tramadol and test sample via administration of naloxone indicate the involvement of opioidergic mechanism. Our compound exhibited significant anti-inflammatory activity in second phase of carrageenan induced paw oedema model. Histological and biochemical parameters exhibited less ulcerogenic potential as compared to aspirin.
Conclusion: Our findings suggests that our test compound has desirable anti-nociceptive and anti-inflammatory potentials with less propensity to cause gastric ulcer.
Keywords: NSAIDs; gastric ulcer; hot plate; inflammation; nociception.
© 2022 Rahman et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures





References
-
- Laveti D, Kumar M, Hemalatha R, et al. Anti-inflammatory treatments for chronic diseases: a review. Inflamm Allergy Drug Targets. 2013;12:349–361. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

