The Capacity to Repair Sperm DNA Damage in Zygotes is Enhanced by Inhibiting WIP1 Activity
- PMID: 35478962
- PMCID: PMC9037036
- DOI: 10.3389/fcell.2022.841327
The Capacity to Repair Sperm DNA Damage in Zygotes is Enhanced by Inhibiting WIP1 Activity
Abstract
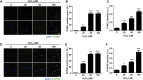
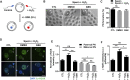
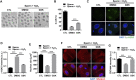
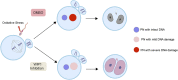
Maintaining genome integrity in germ cells is essential not only for successful fertilization and embryo development, but also to ensure proper transmission of genetic information across generations. However, unlike oocytes, sperm are incapable of repairing DNA damage. Therefore, sperm DNA damage is repaired after fertilization in zygotes using maternal DNA repair factors. In this study, we found that zygotic repair of paternal DNA damage is enhanced by inhibiting WIP1 activity. Oxidative stress induced DNA damage in sperm and severely impaired motility. Although DNA damage in sperm did not compromise fertilization, it increased DNA damage in the paternal pronucleus of zygotes. However, WIP1 inhibition during fertilization reduced DNA damage in the paternal pronucleus, improving the rate of two-cell development, and subsequent zygotic genome activation. Therefore, our results suggest that WIP1 inhibition could enhance maternal DNA repair capacity and thereby decrease paternal DNA damage in zygotes.
Keywords: DNA repair; WIP1; sperm dna damage; zygote; zygotic genome activation.
Copyright © 2022 Leem, Bai and Oh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

