Multinucleated Giant Cells: Current Insights in Phenotype, Biological Activities, and Mechanism of Formation
- PMID: 35478968
- PMCID: PMC9035892
- DOI: 10.3389/fcell.2022.873226
Multinucleated Giant Cells: Current Insights in Phenotype, Biological Activities, and Mechanism of Formation
Abstract
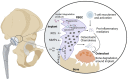
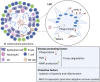
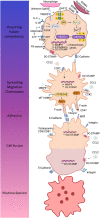
Monocytes and macrophages are innate immune cells with diverse functions ranging from phagocytosis of microorganisms to forming a bridge with the adaptive immune system. A lesser-known attribute of macrophages is their ability to fuse with each other to form multinucleated giant cells. Based on their morphology and functional characteristics, there are in general three types of multinucleated giant cells including osteoclasts, foreign body giant cells and Langhans giant cells. Osteoclasts are bone resorbing cells and under physiological conditions they participate in bone remodeling. However, under pathological conditions such as rheumatoid arthritis and osteoporosis, osteoclasts are responsible for bone destruction and bone loss. Foreign body giant cells and Langhans giant cells appear only under pathological conditions. While foreign body giant cells are found in immune reactions against foreign material, including implants, Langhans giant cells are associated with granulomas in infectious and non-infectious diseases. The functionality and fusion mechanism of osteoclasts are being elucidated, however, our knowledge on the functions of foreign body giant cells and Langhans giant cells is limited. In this review, we describe and compare the phenotypic aspects, biological and functional activities of the three types of multinucleated giant cells. Furthermore, we provide an overview of the multinucleation process and highlight key molecules in the different phases of macrophage fusion.
Keywords: Langhans giant cell (LGC); cell fusion; foreign body giant cell (FBGC); macrophage; migration; multinucleated giant cell (MGC); multinucleation; osteoclast.
Copyright © 2022 Ahmadzadeh, Vanoppen, Rose, Matthys and Wouters.
Conflict of interest statement
CW obtained unrestricted grants to KU Leuven from Novartis, Roche, GSK immuno-inflammation and Pfizer. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Aghbali A., Rafieyan S., Mohamed-Khosroshahi L., Baradaran B., Shanehbandi D., Kouhsoltani M. (2016). IL-4 Induces the Formation of Multinucleated Giant Cells and Expression of β5 Integrin in central Giant Cell Lesion. Med. Oral Patol Oral Cir Bucal. 22 (1), e1–e6. 10.4317/medoral.20935 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

