Preparation and application of layered double hydroxide nanosheets
- PMID: 35479011
- PMCID: PMC9036865
- DOI: 10.1039/d1ra03289e
Preparation and application of layered double hydroxide nanosheets
Abstract
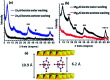
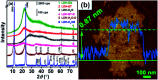
Layered double hydroxides (LDH) with unique structure and excellent properties have been widely studied in recent years. LDH have found widespread applications in catalysts, polymer/LDH nanocomposites, anion exchange materials, supercapacitors, and fire retardants. The exfoliated LDH ultrathin nanosheets with a thickness of a few atomic layers enable a series of new opportunities in both fundamental research and applications. In this review, we mainly summarize the LDH exfoliation methods developed in recent years, the recent developments for the direct synthesis of LDH single-layer nanosheets, and the applications of LDH nanosheets in catalyzing oxygen evolution reactions, crosslinkers, supercapacitors and delivery carriers.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures































References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

