Contact-Dependent Granzyme B-Mediated Cytotoxicity of Th17-Polarized Cells Toward Human Oligodendrocytes
- PMID: 35479072
- PMCID: PMC9035748
- DOI: 10.3389/fimmu.2022.850616
Contact-Dependent Granzyme B-Mediated Cytotoxicity of Th17-Polarized Cells Toward Human Oligodendrocytes
Abstract
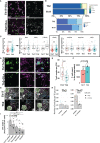
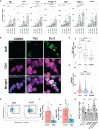
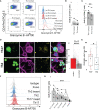
Multiple sclerosis (MS) is characterized by the loss of myelin and of myelin-producing oligodendrocytes (OLs) in the central nervous system (CNS). Pro-inflammatory CD4+ Th17 cells are considered pathogenic in MS and are harmful to OLs. We investigated the mechanisms driving human CD4+ T cell-mediated OL cell death. Using fluorescent and brightfield in vitro live imaging, we found that compared to Th2-polarized cells, Th17-polarized cells show greater interactions with primary human OLs and human oligodendrocytic cell line MO3.13, displaying longer duration of contact, lower mean speed, and higher rate of vesicle-like structure formation at the sites of contact. Using single-cell RNA sequencing, we assessed the transcriptomic profile of primary human OLs and Th17-polarized cells in direct contact or separated by an insert. We showed that upon close interaction, OLs upregulate the expression of mRNA coding for chemokines and antioxidant/anti-apoptotic molecules, while Th17-polarized cells upregulate the expression of mRNA coding for chemokines and pro-inflammatory cytokines such as IL-17A, IFN-γ, and granzyme B. We found that secretion of CCL3, CXCL10, IFN-γ, TNFα, and granzyme B is induced upon direct contact in cocultures of human Th17-polarized cells with human OLs. In addition, we validated by flow cytometry and immunofluorescence that granzyme B levels are upregulated in Th17-polarized compared to Th2-polarized cells and are even higher in Th17-polarized cells upon direct contact with OLs or MO3.13 cells compared to Th17-polarized cells separated from OLs by an insert. Moreover, granzyme B is detected in OLs and MO3.13 cells following direct contact with Th17-polarized cells, suggesting the release of granzyme B from Th17-polarized cells into OLs/MO3.13 cells. To confirm granzyme B-mediated cytotoxicity toward OLs, we showed that recombinant human granzyme B can induce OLs and MO3.13 cell death. Furthermore, pretreatment of Th17-polarized cells with a reversible granzyme B blocker (Ac-IEPD-CHO) or a natural granzyme B blocker (serpina3N) improved survival of MO3.13 cells upon coculture with Th17 cells. In conclusion, we showed that human Th17-polarized cells form biologically significant contacts with human OLs and exert direct toxicity by releasing granzyme B.
Keywords: CD4 T lymphocytes; Th17 cells; granzyme B; human oligodendrocytes; multiple sclerosis; neuroinflammation.
Copyright © 2022 Jamann, Cui, Desu, Pernin, Tastet, Halaweh, Farzam-kia, Mamane, Ouédraogo, Cleret-Buhot, Daigneault, Balthazard, Klement, Lemaître, Arbour, Antel, Stratton and Larochelle.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

