A Capsid Virus-Like Particle-Based SARS-CoV-2 Vaccine Induces High Levels of Antibodies and Protects Rhesus Macaques
- PMID: 35479095
- PMCID: PMC9037084
- DOI: 10.3389/fimmu.2022.857440
A Capsid Virus-Like Particle-Based SARS-CoV-2 Vaccine Induces High Levels of Antibodies and Protects Rhesus Macaques
Abstract
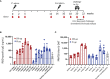
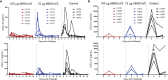
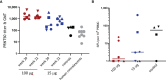
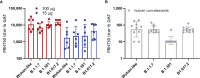
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a worldwide pandemic. Here, we present non-human primate immunogenicity and protective efficacy data generated with the capsid virus-like particle (cVLP)-based vaccine ABNCoV2 that has previously demonstrated immunogenicity in mice. In rhesus macaques, a single vaccination with either 15 or 100 μg ABNCoV2 induced binding and neutralizing antibodies in a dose-dependent manner, at levels comparable to those measured in human convalescents. A second vaccine administration led to a >50-fold increase in neutralizing antibodies, with 2-log higher mean levels in the 100-μg ABNCoV2 group compared with convalescent samples. Upon SARS-CoV-2 challenge, a significant reduction in viral load was observed for both vaccine groups relative to the challenge control group, with no evidence of enhanced disease. Remarkably, neutralizing antibody titers against an original SARS-CoV-2 isolate and against variants of concern were comparable, indicating a potential for broad protection afforded by ABNCoV2, which is currently in clinical testing.
Keywords: COVID-19; SARS-CoV-2; broad neutralizing antibodies; corona; non-human primates (NHP); protection; variants of concern (VOCs); virus-like particles (VLP).
Copyright © 2022 Volkmann, Koopman, Mooij, Verschoor, Verstrepen, Bogers, Idorn, Paludan, Vang, Nielsen, Sander, Schmittwolf, Hochrein and Chaplin.
Conflict of interest statement
AS are listed as co-inventors on a patent application covering the AP205 CLP vaccine platform technology (WO2016112921 A1). AV, CS, HH, and PC are employees of Bavarian Nordic, a vaccine development company that has licensed ABNCoV2 for further development and commercialization. MN and AS were employed by AdaptVac Aps. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- WHO . WHO Coronavirus (COVID-19) Dashboard 2021. World Health Organization; (2021).
-
- Scobie H, CDC . Update on Emerging SARS-CoV-2 Variants and Vaccine Considerations. In: Coronavirus Disease 2019 (COVID-19) Vaccines. May 12, 2021; ACIP Meeting May 12, 2021. Centers for Disease Control and Prevention (CDC) (2021).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

