Diverse and efficient catalytic applications of new cockscomb flower-like Fe3O4@SiO2@KCC-1@MPTMS@CuII mesoporous nanocomposite in the environmentally benign reduction and reductive acetylation of nitroarenes and one-pot synthesis of some coumarin compounds
- PMID: 35479105
- PMCID: PMC9020196
- DOI: 10.1039/d1ra08763k
Diverse and efficient catalytic applications of new cockscomb flower-like Fe3O4@SiO2@KCC-1@MPTMS@CuII mesoporous nanocomposite in the environmentally benign reduction and reductive acetylation of nitroarenes and one-pot synthesis of some coumarin compounds
Abstract
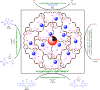
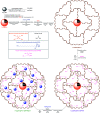
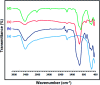
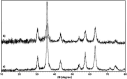
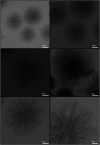
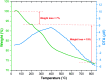
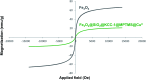
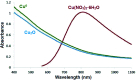
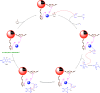
In this research, Fe3O4@SiO2@KCC-1@MPTMS@CuII as a new cockscomb flower-like mesoporous nanocomposite was prepared and characterized by various techniques including Fourier transform infrared (FT-IR) spectroscopy, powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), SEM-based energy-dispersive X-ray (EDX) spectroscopy, inductively coupled plasma-optical emission spectrometry (ICP-OES), thermogravimetric analysis/differential thermal analysis (TGA/DTA), vibrating sample magnetometry (VSM), UV-Vis spectroscopy, and Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halenda (BJH) analyses. The as-prepared Fe3O4@SiO2@KCC-1@MPTMS@CuII mesoporous nanocomposite exhibited satisfactory catalytic activity in the reduction and reductive acetylation of nitroarenes in a water medium and solvent-free one-pot synthesis of some coumarin compounds including 3,3'-(arylmethylene)bis(4-hydroxy-2H-chromen-2-ones) (namely, bis-coumarins) (3a-n) and 2-amino-4-aryl-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitriles (6a-n) along with acceptable turnover numbers (TONs) and turnover frequencies (TOFs). Furthermore, the mentioned CuII-containing mesoporous nanocatalyst was conveniently recovered by a magnet from reaction environments and reused for at least seven cycles without any significant loss in activity, which confirms its good stability.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
NiII-containing l-glutamic acid cross-linked chitosan anchored on Fe3O4/f-MWCNT: a sustainable catalyst for the green reduction and one-pot two-step reductive Schotten-Baumann-type acetylation of nitroarenes.Nanoscale Adv. 2024 Jun 25;6(15):3961-3977. doi: 10.1039/d4na00160e. eCollection 2024 Jul 23. Nanoscale Adv. 2024. PMID: 39050942 Free PMC article.
-
NiII NPs entrapped within a matrix of l-glutamic acid cross-linked chitosan supported on magnetic carboxylic acid-functionalized multi-walled carbon nanotube: a new and efficient multi-task catalytic system for the green one-pot synthesis of diverse heterocyclic frameworks.RSC Adv. 2022 Jun 7;12(26):16454-16478. doi: 10.1039/d1ra08454b. eCollection 2022 Jun 1. RSC Adv. 2022. PMID: 35754864 Free PMC article.
-
A new strategy for immobilization of copper on the Fe3O4@EDTA nanocomposite and its efficient catalytic applications in reduction and one-pot reductive acetylation of nitroarenes and also N-acetylation of arylamines.Heliyon. 2024 Jul 26;10(15):e35062. doi: 10.1016/j.heliyon.2024.e35062. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39166007 Free PMC article.
-
A copper(ii) complex containing pyridine-2-carbaldehyde and its direct binding onto ethylenediamine functionalized with Fe3O4@SiO2 nanoparticles for catalytic applications.RSC Adv. 2023 Oct 4;13(42):29121-29140. doi: 10.1039/d3ra05649j. eCollection 2023 Oct 4. RSC Adv. 2023. PMID: 37800129 Free PMC article.
-
Functionalization of Magnetic UiO-66-NH2 with a Chiral Cu(l-proline)2 Complex as a Hybrid Asymmetric Catalyst for CO2 Conversion into Cyclic Carbonates.Inorg Chem. 2024 Apr 1;63(13):6051-6066. doi: 10.1021/acs.inorgchem.4c00376. Epub 2024 Mar 19. Inorg Chem. 2024. PMID: 38501387
Cited by
-
NiII-containing l-glutamic acid cross-linked chitosan anchored on Fe3O4/f-MWCNT: a sustainable catalyst for the green reduction and one-pot two-step reductive Schotten-Baumann-type acetylation of nitroarenes.Nanoscale Adv. 2024 Jun 25;6(15):3961-3977. doi: 10.1039/d4na00160e. eCollection 2024 Jul 23. Nanoscale Adv. 2024. PMID: 39050942 Free PMC article.
-
NiII NPs entrapped within a matrix of l-glutamic acid cross-linked chitosan supported on magnetic carboxylic acid-functionalized multi-walled carbon nanotube: a new and efficient multi-task catalytic system for the green one-pot synthesis of diverse heterocyclic frameworks.RSC Adv. 2022 Jun 7;12(26):16454-16478. doi: 10.1039/d1ra08454b. eCollection 2022 Jun 1. RSC Adv. 2022. PMID: 35754864 Free PMC article.
-
Magnetically recoverable Fe3O4@chitosan@Ni2B: a bio-based catalyst for one-pot green and efficient synthesis of tetrahydrobenzo[b]pyrans.Nanoscale Adv. 2025 May 9;7(12):3701-3721. doi: 10.1039/d4na01020e. eCollection 2025 Jun 10. Nanoscale Adv. 2025. PMID: 40352460 Free PMC article.
-
Mesoporous Calcium-Silicate Nanoparticles Loaded with Prussian Blue Promotes Enterococcus Faecalis Ferroptosis-Like Death by Regulating Bacterial Redox Pathway ROS/GSH.Int J Nanomedicine. 2022 Nov 4;17:5187-5205. doi: 10.2147/IJN.S382928. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36388876 Free PMC article.
-
Immobilized nickel boride nanoparticles on magnetic functionalized multi-walled carbon nanotubes: a new nanocomposite for the efficient one-pot synthesis of 1,4-benzodiazepines.Nanoscale Adv. 2023 Aug 9;5(17):4499-4520. doi: 10.1039/d3na00415e. eCollection 2023 Aug 24. Nanoscale Adv. 2023. PMID: 37638163 Free PMC article.
References
-
- Erythropel H. C. Zimmerman J. B. de Winter T. M. Petitjean L. Melnikov F. Lam C. H. Lounsbury A. W. Mellor K. E. Janković N. Z. Tu Q. Pincus L. N. Falinski M. M. Shi W. Coish P. Plata D. L. Anastas P. A. Green Chem. 2018;20:1929–1961.
- Pérez-Venegas M. Juaristi E. ACS Sustainable Chem. Eng. 2020;8:8881–8893.
- Mousavi H. Int. J. Bio. Macromol. 2021;186:1003–1166. - PubMed
- Rimaz M. Mousavi H. Khalili B. Aali F. J. Chin. Chem. Soc. 2018;65:1389–1397.
- Rimaz M. Mousavi H. Behnam M. Khalili B. Curr. Chem. Lett. 2016;5:145–154.
- Rimaz M. Jalalian Z. Mousavi H. Prager R. H. Tetrahedron Lett. 2016;57:105–109.
- Nivetha N. Thangamani A. J. Mol. Struct. 2021;1242:130716.
-
- Li A. Y. Moores A. ACS Sustainable Chem. Eng. 2019;7:10182–10197.
- Hutching G. J. ACS Cent. Sci. 2018;4:1095–1101. - PMC - PubMed
- Anastas P. T. Kirchhoff M. M. Williamson T. C. Appl. Catal., A. 2001;221:3–13.
- Centi G. Perathoner S. Top. Catal. 2009;52:948–961.
- Lamb A. C. Lee A. F. Wilson K. Aust. J. Chem. 2020;73:832–852.
-
- Goodman E. D. Zhao C. Cargnello M. ACS Cent. Sci. 2020;6:1916–1937. - PMC - PubMed
- Schlögl R. Angew. Chem., Int. Ed. 2015;54:3465–3520. - PubMed
- Lung R. Du X. Huang Y. Jiang X. Zhang Q. Guo Y. Liu K. Qiao B. Wang A. Zhang T. Chem. Rev. 2020;120:11986–12043. - PubMed
- Lippi R. Coghlan C. J. Howard S. C. Easton C. D. Gu Q. Patel J. Sumby C. J. Kennedy D. F. Dooman C. J. Aust. J. Chem. 2020;73:1271–1283.
- Tadon N. Patil S. M. Tadon R. Kumar P. RSC Adv. 2021;11:21291–21300. - PMC - PubMed
-
- Polshetiwar V. Luque R. Fihri A. Zhu H. Bouhara M. Basset J.-M. Chem. Rev. 2011;111:3036–3075. - PubMed
- Shylesh S. Schünemann V. Thiel W. R. Angew. Chem., Int. Ed. 2010;49:3428–3459. - PubMed
- Wang D. Astruc D. Chem. Rev. 2014;114:6949–6985. - PubMed
- Gawande M. B. Luque R. Zboril R. ChemCatChem. 2014;6:3312–3313.
- Karimi B. Mansouri F. Mirzaei H. M. ChemCatChem. 2015;7:1736–1789.
- Mohammadi Ziarani G. Kheilkordi Z. Mohajer F. Badiei A. Luque R. RSC Adv. 2021;11:17456–17477. - PMC - PubMed
- Pawar A. Gajare S. Patil A. Kurane R. Rashinkar G. Patil S. Res. Chem. Intermed. 2021;47:2801–2820.
- Aghaei-Hashjin M. Yahyazadeh A. Abbaspour-Gilandeh E. RSC Adv. 2021;11:23491–23505. - PMC - PubMed
- Mohammadsaleh F. Dehdashti Jahromi M. Hajipour A. R. Hosseini S. M. Niknam K. RSC Adv. 2021;11:20812–20823. - PMC - PubMed
- Hosseini Mohtasham N. Gholizadeh M. Res. Chem. Intermed. 2021;47:2507–2525.
-
- Maity A. Polshettiwar V. ChemSusChem. 2017;10:3866–3913. - PMC - PubMed
- Huang X. Tao Z. Praskavich J. C. Goswami A. Al-Sharab J. F. Minko T. Polshettiwar V. Asefa T. Langmuir. 2014;30:10886–10898. - PubMed
- Shaban M. Hasanzadeh M. RSC Adv. 2020;10:37116–37133. - PMC - PubMed
- Hao P. Peng B. Shan B.-Q. Yang T.-Q. Zhang K. Nanoscale Adv. 2020;2:1792–1810. - PMC - PubMed
- Wang A. Du X. Liu Z. Shi S. Lv H. J. Mater. Chem. A. 2019;7:5111–5152.
LinkOut - more resources
Full Text Sources
Miscellaneous

