TCCA-mediated oxidative rearrangement of tetrahydro-β-carbolines: facile access to spirooxindoles and the total synthesis of (±)-coerulescine and (±)-horsfiline
- PMID: 35479130
- PMCID: PMC9031260
- DOI: 10.1039/d1ra02381k
TCCA-mediated oxidative rearrangement of tetrahydro-β-carbolines: facile access to spirooxindoles and the total synthesis of (±)-coerulescine and (±)-horsfiline
Abstract
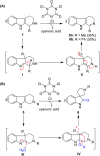
Multi-reactive centered reagents are beneficial in chemical synthesis due to their advantage of minimal material utilization and formation of less by-products. Trichloroisocyanuric acid (TCCA), a reagent with three reactive centers, was employed in the synthesis of spirooxindoles through the oxidative rearrangement of various N-protected tetrahydro-β-carbolines. In this protocol, low equivalents of TCCA were required to access spirooxindoles (up to 99% yield) with a wide substrate scope. Furthermore, the applicability and robustness of this protocol were proven for the gram-scale total synthesis of natural alkaloids such as (±)-coerulescine (1) and (±)-horsfiline (2) in excellent yields.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Ye N. Chen H. Wold E. A. Shi P. Y. Zhou J. ACS Infect. Dis. 2016;2:382–392. doi: 10.1021/acsinfecdis.6b00041. - DOI - PMC - PubMed
- Zhou B. Yang Y. Shi J. Luo Z. Li Y. J. Org. Chem. 2013;78:2897–2907. doi: 10.1021/jo302655u. - DOI - PubMed
- Li C. Chan C. Heimann A. C. Danishefsky S. J. Angew. Chem., Int. Ed. 2007;46:1444–1447. doi: 10.1002/anie.200604071. - DOI - PubMed
- White J. D. Li Y. Ihle D. C. J. Org. Chem. 2010;75:3569–3577. doi: 10.1021/jo1002714. - DOI - PubMed
-
- Mei G. J. Shi F. Chem. Commun. 2018;54:6607–6621. doi: 10.1039/C8CC02364F. - DOI - PubMed
- Pelay-Gimeno M. Glas A. Koch O. Grossmann T. N. Angew. Chem., Int. Ed. 2015;54:8896–8927. doi: 10.1002/anie.201412070. - DOI - PMC - PubMed
- Bhaskar G. Arun Y. Balachandran C. Saikumar C. Perumal P. T. Eur. J. Med. Chem. 2012;51:79–91. doi: 10.1016/j.ejmech.2012.02.024. - DOI - PubMed
-
- Barakat A. Shahidul Islam M. Mansur Ghawas H. Mohammed Al-Majid A. El-Senduny F. F. Badria F. A. Elshaier Y. A. M. M. Ghabbour H. A. RSC Adv. 2018;8:14335–14346. doi: 10.1039/C8RA02358A. - DOI - PMC - PubMed
- Yu B. Zheng Y.-C. Shi X.-J. Qi P.-P. Liu H.-M. Anti-Cancer Agents Med. Chem. 2016;16:1315–1324. doi: 10.2174/1871520615666151102093825. - DOI - PubMed
- Chen L. Xie J. Song H. Liu Y. Gu Y. Wang L. Wang Q. J. Agric. Food Chem. 2016;64:6508–6516. doi: 10.1021/acs.jafc.6b02683. - DOI - PubMed
- Senwar K. R. Sharma P. Reddy T. S. Jeengar M. K. Nayak V. L. Naidu V. G. M. Kamal A. Shankaraiah N. Eur. J. Med. Chem. 2015;102:413–424. doi: 10.1016/j.ejmech.2015.08.017. - DOI - PubMed
-
- El-Hashash M. A. Rizk S. A. J. Heterocycl. Chem. 2017;54:1776–1784. doi: 10.1002/jhet.2758. - DOI
LinkOut - more resources
Full Text Sources

