UV-visible light-induced photochemical synthesis of benzimidazoles by coomassie brilliant blue coated on W-ZnO@NH2 nanoparticles
- PMID: 35479136
- PMCID: PMC9030167
- DOI: 10.1039/d0ra10843j
UV-visible light-induced photochemical synthesis of benzimidazoles by coomassie brilliant blue coated on W-ZnO@NH2 nanoparticles
Abstract
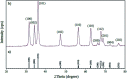
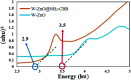
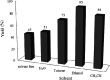
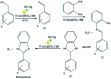
Heterogeneous photocatalysts proffer a promising method to actualize eco-friendly and green organic transformations. Herein, a new photochemical-based methodology is disclosed in the preparation of a wide range of benzimidazoles through condensation of o-phenylenediamine with benzyl alcohols in the air under the illumination of an HP mercury lamp in the absence of any oxidizing species catalyzed by a new photocatalyst W-ZnO@NH2-CBB. In this photocatalyst, coomassie brilliant blue (CBB) is heterogenized onto W-ZnO@NH2 to improve the surface characteristics at the molecular level and enhance the photocatalytic activity of both W-ZnO@NH2 and CBB fragments. This unprecedented heterogeneous nanocatalyst is also identified by means of XRD, FT-IR, EDS, TGA-DTG, and SEM. The impact of some influencing parameters on the synthesis route and effects on the catalytic efficacy of W-ZnO@NH2-CBB are also assessed. The appropriate products are attained for both the electron-withdrawing and electron-donating substituents in the utilized aromatic alcohols. Furthermore, preparation of benzimidazoles is demonstrated to occur mainly via a radical mechanism, which shows that reactive species such as ·O2 -, OH˙ and h+ would be involved in the photocatalytic process. Stability and reusability studies also warrant good reproducibility of the nanophotocatalyst for at least five runs. Eventually, a hot filtration test proved that the nanohybrid photocatalyst is stable in the reaction medium. Using an inexpensive catalyst, UV-vis light energy and air, as a low cost and plentiful oxidant, puts this methodology in the green chemistry domain and energy-saving organic synthesis strategies. Finally, the anticancer activity of W-ZnO nanoparticles is investigated on MCF7 breast cancer cells by MTT assay. This experiment reveals that the mentioned nanoparticles have significant cytotoxicity towards the selected cell line.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

















References
-
- Sahoo S. K. Renewable Sustainable Energy Rev. 2016;59:927–939. doi: 10.1016/j.rser.2016.01.049. - DOI
-
- Shanmugam S. Xu S. Adnan N. N. M. Boyer C. Macromolecules. 2018;51:779–790. doi: 10.1021/acs.macromol.7b02215. - DOI
-
- Li Z. Song H. Guo R. Zuo M. Hou C. Sun S. He X. Sun Z. Chu W. Green Chem. 2019;21:3602–3605. doi: 10.1039/C9GC01359H. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

