Adsorption of lindane (γ-hexachlorocyclohexane) on nickel modified graphitic carbon nitride: a theoretical study
- PMID: 35479347
- PMCID: PMC9034011
- DOI: 10.1039/d1ra03797h
Adsorption of lindane (γ-hexachlorocyclohexane) on nickel modified graphitic carbon nitride: a theoretical study
Abstract
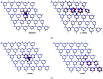
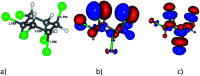
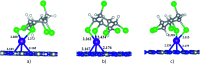
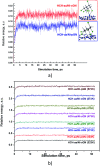
Adsorption of lindane (HCH) on nickel modified graphitic carbon nitride (Ni-gCN) was investigated using a novel, accurate and broadly parametrized self-consistent tight-binding quantum chemical (GFN2-xTB) method. Two graphitic carbon nitride (gCN) models were used: corrugated and planar, which represent the material with different thicknesses. Electronic properties of the adsorbates and adsorbent were estimated via vertical ionization potential, vertical electron affinity, global electrophilicity index and the HOMO and LUMO. Adsorption energy and population analyses were carried out to figure out the nature of the adsorption process. The results reveal that the introduction of the nickel atom significantly influences the electronic properties of gCN, and results in the improvement of adsorption ability of gCN for lindane. Lindane adsorption on Ni-gCN is considered as chemisorption, which is primarily supported by the interaction of the nickel atom and chlorine atoms of HCH. The effect of solvents (water, ethanol, acetonitrile) was investigated via the analytical linearized Poisson-Boltzmann model. Due to the strong chemisorption, Ni-gCN can collect lindane from different solvents. The adsorption configurations of HCH on Ni-gCN were also shown to be thermally stable at 298 K, 323 K, 373 K, 473 K, and 573 K via molecular simulation calculations. The obtained results are useful for a better understanding of lindane adsorption on Ni-gCN and for the design of materials with high efficiency for lindane treatment based on adsorption-photocatalytic technology.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

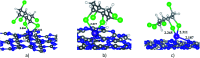

References
-
- Persistent Organic Pollutants (POPs) and Pesticides | The Caribbean Environment Programme (CEP), https://www.unep.org/cep/persistent-organic-pollutants-pops-and-pesticides, accessed 15 April 2021
-
- UNTC, https://treaties.un.org/Pages/ViewDetails.aspx?src=IND&mtdsg_no=XXVII-15..., accessed 15 April 2021
-
- Nayyar N. Sangwan N. Kohli P. Verma H. Kumar R. Negi V. Oldach P. Mahato N. K. Gupta V. Lal R. Rev. Environ. Health. 2014;29:49–52. - PubMed
-
- Oturan M. A. Aaron J. J. Crit. Rev. Environ. Sci. Technol. 2014;44:2577–2641. doi: 10.1080/10643389.2013.829765. - DOI
LinkOut - more resources
Full Text Sources

