A three-dimensional nano-network WO3/F-TiO2-{001} heterojunction constructed with OH-TiOF2 as the precursor and its efficient degradation of methylene blue
- PMID: 35479479
- PMCID: PMC9037076
- DOI: 10.1039/d1ra04809k
A three-dimensional nano-network WO3/F-TiO2-{001} heterojunction constructed with OH-TiOF2 as the precursor and its efficient degradation of methylene blue
Abstract
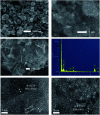
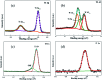
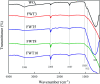
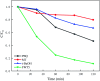
In this study, three-dimensional nested WO3/F-TiO2-{001} photocatalysts with different WO3 loadings were prepared by a hydrothermal process and used to degrade methylene blue (MB). The photocatalysts with various ratios of WO3 to OH-TiOF2 can be transformed into a three-dimensional network WO3/F-TiO2 hetero-structure with {001} surface exposure. The results showed that the composite catalyst with 5% WO3, denoted as FWT5, had the best comprehensive degradation effect. FWT5 has a limited band gap of 2.9 eV, which can be used as an advanced photocatalyst to respond to sunlight and degrade MB. The average pore diameter of the composite catalyst is 10.3 nm, and the multi-point specific surface area is 56 m2 g-1. Compared with pure TiOF2, the average pore size of the composite catalyst decreased by 8.44 nm and the specific surface area increased by 51.2 m2 g-1, which provides a larger contact space for the catalytic components and pollutants. Moreover, TiO2 on the {001} surface has higher photocatalytic activity and methylene blue can be better degraded. Under the irradiation of 0.03 g FWT5 composite catalyst with a simulated solar light source for 2 h, the degradation rate of 10 mg L-1 methylene blue can reach 82.9%. The trapping experiment showed that photo-generated holes were the principal functional component of WO3/F-TiO2-{001} photo-catalysis, which could capture OH- and form hydroxyl radical (˙OH) and improved the photocatalytic degradation performance. Kinetic studies show that the photocatalytic degradation of MB fits with the quasi-first order kinetic model.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Popa N. Visa M. Adv. Powder Technol. 2017;28:1866–1876. doi: 10.1016/j.apt.2017.04.014. - DOI
-
- Zhang S. Li H. Yang Z. J. Alloys Compd. 2017;722:555–563. doi: 10.1016/j.jallcom.2017.06.095. - DOI
-
- Han W. Wang Y. Zheng Y. Adv. Mater. Res. 2008;47:1438–1441.
-
- Fu Y. Jin Z. Ni Y. Du H. Wang T. Thin Solid Films. 2009;517:5634–5640. doi: 10.1016/j.tsf.2009.02.030. - DOI
-
- Sandoval A. Ventura C. H. Klimova T. E. Fuel. 2017;198:22–30. doi: 10.1016/j.fuel.2016.11.007. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

