Dupilumab-associated cicatrizing ocular disease
- PMID: 35479518
- PMCID: PMC9035391
- DOI: 10.1016/j.ajoc.2022.101528
Dupilumab-associated cicatrizing ocular disease
Abstract
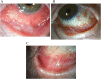
Purpose: To describe three cases of bilateral cicatrizing conjunctivitis associated with dupilumab treatment for atopic dermatitis.
Observations: Case 1 is a 69 year-old male with a history of mild, stable cicatrizing conjunctivitis thought to be secondary to atopic dermatitis. His cicatrizing disease progressed significantly after starting dupilumab, and then stabilized after dupilumab was discontinued. Case 2 is a 72 year-old male who presented with bilateral cicatrizing conjunctivitis. His symptoms of ocular erythema and irritation started shortly after initiating dupilumab for atopic dermatitis. The dupilumab was discontinued and the patient's symptoms resolved and ocular surface scarring stabilized. Case 3 is a 47 year-old male with a history of allergic conjunctivitis who was found to have new onset unilateral symblepharon approximately 12 months after starting dupilumab for atopic dermatitis. The dupilumab was discontinued and his ocular disease stabilized. However, his cutaneous symptoms worsened significantly, so dupilumab was restarted at half the previous dose. His atopic dermatitis symptoms improved and cicatrizing conjunctivitis remained stable on this regimen.
Conclusions and importance: Cicatrizing conjunctivitis with symblepharon formation distinct from ocular cicactricial pemphigoid is a potential side effect of dupilumab therapy for atopic dermatitis. Progression of the cicatrization appears to halt with discontinuation, or potentially dose reduction, of dupilumab.
Keywords: Atopic dermatitis; Cicatrizing conjunctivitis; Dupilumab; Pseudopemphigoid.
© 2022 The Authors.
Conflict of interest statement
No financial disclosure or conflicting relationship exists for any author.
Figures
References
-
- Blauvelt A., de Bruin-Weller M., Gooderham M., et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. Jun 10 2017;389(10086):2287–2303. doi: 10.1016/S0140-6736(17)31191-1. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

