Design, synthesis and cytotoxic evaluation of a library of oxadiazole-containing hybrids
- PMID: 35479556
- PMCID: PMC9040754
- DOI: 10.1039/d1ra05602f
Design, synthesis and cytotoxic evaluation of a library of oxadiazole-containing hybrids
Abstract
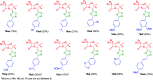
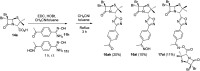
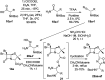
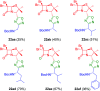
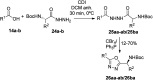
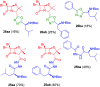
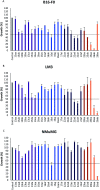
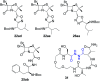
The development of hybrid compounds led to the discovery of new pharmacologically active agents for some of the most critical diseases, including cancer. Herein, we describe a new series of oxadiazole-containing structures designed by a molecular hybridization approach. Penicillin derivatives and amino acids were linked to amino acid and aromatic moieties through the formation of a 1,2,4-oxadiazole ring. Alternatively, condensation between amino acid-derived hydrazides and an activated penicillanic acid led to a series of 1,3,4-oxadiazole penicillin-containing hybrids and non-cyclized diacylhydrazides. From the cytotoxicity assays it is highlighted that two 1,2,4-oxadiazoles and one 1,3,4-oxadiazole connecting a penicillin and aliphatic amino acids displayed a high degree of cytotoxic selectivity, ranging between being three and four times more potent against tumor cells than normal cells. The results give a very interesting perspective suggesting that these hybrid compounds can offer a novel antitumor scaffold with promising cytotoxicity profiles.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Galloway W. R. J. D. Isidro-Llobet A. Spring D. R. Nat. Commun. 2010;1:1–13. doi: 10.1038/ncomms1081. - DOI - PubMed
- O' Connor C. J. Laraia L. Spring D. R. Chem. Soc. Rev. 2011;40:4332–4345. doi: 10.1039/C1CS15053G. - DOI - PubMed
- Beckmann H. O' Connor C. J. Spring D. R. Chem. Soc. Rev. 2012;41:4444–4456. doi: 10.1039/C2CS35023H. - DOI - PubMed
- Mateu N., Kidd S. L., Osberger T. J., Stewart H. L., Bartlett S., Galloway W. R., North A. J. P., Sore H. F. and Spring D. R., The Application of Diversity-oriented Synthesis in Chemical Biology. in Chemical and Biological Synthesis: Enabling Approaches for Understanding Biology, ed. N. J. Westwood and A. Nelson, The Royal Society of Chemistry, Cambridge, 2018, pp. 8–44
-
- Eicher T., Hauptmann S. and Speicher A., The Chemistry of Heterocycles, Wiley-VCH Verlag GmbH & Co, Weinheim, 2nd edn, 2003
-
- Li Z. Chen W. Hale J. J. Lynch C. L. Mills S. G. Hajdu R. Keohane C. A. Rosenbach M. J. Milligam J. A. Shein G. J. Chrebet G. Parent S. A. Bergstrom J. Card D. Forrest M. Quackenbush E. J. Wickham L. A. Vargas H. Evans R. M. Rosen H. Mandala S. J. J. Med. Chem. 2005;48:6169–6173. doi: 10.1021/jm0503244. - DOI - PubMed
LinkOut - more resources
Full Text Sources

