DDQ as a versatile and easily recyclable oxidant: a systematic review
- PMID: 35479576
- PMCID: PMC9040906
- DOI: 10.1039/d1ra04575j
DDQ as a versatile and easily recyclable oxidant: a systematic review
Abstract
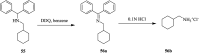
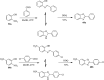
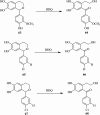
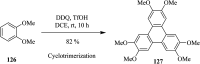
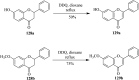
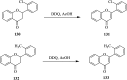
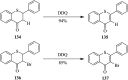
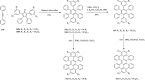
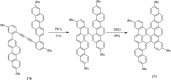
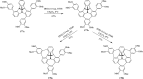
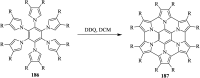
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) is the most widely used quinone with a high reduction potential, and it commonly mediates hydride transfer reactions and shows three accessible oxidation states: quinone (oxidized), semiquinone (one-electron-reduced), and hydroquinone (two-electron-reduced). DDQ has found broad utility as a stoichiometric oxidant in the functionalization of activated C-H bonds and the dehydrogenation of saturated C-C, C-O, and C-N bonds. The cost and toxicity of DDQ triggered recent efforts to develop methods that employ catalytic quantities of DDQ in combination with alternative stoichiometric oxidants. The aerobic catalytic approach was established for the selective oxidation of non-sterically hindered electron-rich benzyl methyl ethers and benzylic alcohols, and effectively extended to the oxidative deprotection of p-methoxybenzyl ethers to generate the alcohols in high selectivity. A combination of DDQ and protic acid is known to oxidize several aromatic donors to the corresponding cation radicals. The excited-state DDQ converts benzyls, heteroarenes, fluoroarenes, benzene, and olefins into their radical cation forms as well as chloride and other anions into their respective radicals. These reactive intermediates have been employed for the generation of C-C and C-X (N, O, or Cl) bonds in the synthesis of valuable natural products and organic compounds. To the best of our knowledge, however, there is still no review article exclusively describing the applications of DDQ in organic synthesis. Therefore, in the present review, we provide an overview of DDQ-induced organic transformations with their scope, limitations and the proposed reaction mechanisms.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





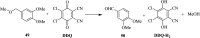
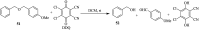
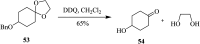
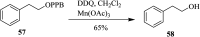
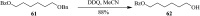

















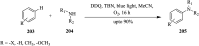

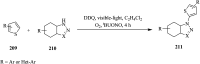
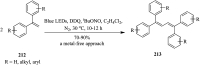
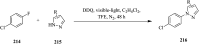


References
-
- Clerici M. G. Ingallina P. Catal. Today. 1998;41:351–364. doi: 10.1016/S0920-5861(98)00025-X. - DOI
-
- Shen Z. Dai J. Xiong J. He X. Mo W. Hu B. Sun N. Hu X. Adv. Synth. Catal. 2011;353:3031–3038. doi: 10.1002/adsc.201100429. - DOI
-
- Bharate S. B. Synlett. 2006;205:0496–0497. doi: 10.1055/s-2006-932456. - DOI
-
- Walker D. Waugh T. D. J. Org. Chem. 1965;30:3240. doi: 10.1021/jo01020a529. - DOI
Publication types
LinkOut - more resources
Full Text Sources

