ANCA Associated Vasculitis Subtypes: Recent Insights and Future Perspectives
- PMID: 35479831
- PMCID: PMC9037725
- DOI: 10.2147/JIR.S284768
ANCA Associated Vasculitis Subtypes: Recent Insights and Future Perspectives
Abstract
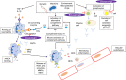
The ANCA associated vasculitides (AAVs) affect a range of internal organs including ear nose and throat, respiratory tract, kidneys, skin and nervous system. They include granulomatosis with polyangiitis (GPA), eosinophilic granulomatosis with polyangiitis (EGPA) and microscopic polyangiitis (MPA). The AAVs are treated with high dose glucocorticoids, immunosuppressants, and targeted biological medications. Since the 1990s classification criteria for the AAVs have been based on clinical features, laboratory tests and basic imaging; an initiative to update the classification criteria incorporating newer tests, for example, anti-neutrophil cytoplasmic antibodies (ANCA) and novel imaging techniques will be published this year. There is also evidence for classification of patients based on ANCA subtype; those with anti-proteinase 3 antibodies (PR3) or anti-myeloperoxidase antibodies (MPO) have differences in response to treatment and clinical outcomes. An update is described within this review. The pathogenesis of AAV involves necrotizing inflammation of small to medium blood vessels involving multiple immunological pathways. We present an update on emerging evidence related to auto-antibodies, complement and lymphocyte pathways. This review describes emerging treatment regimens, including evidence for plasma exchange in severe disease and the inhibitor of the complement C5a receptor (C5aR) inhibitor, Avacopan. Lastly, patient reported outcomes are key secondary outcomes in randomised controlled trials and increasingly clinical practice, we report development in disease specific and glucocorticoid-specific PROs.
Keywords: epidemiology; pathogenesis; patient-reported outcomes; vasculitis.
© 2022 Austin et al.
Conflict of interest statement
Keziah Austin and Shalini Janagan are co-first authors for this study. Dr Joanna C Robson reports grants and/or personal fees from Vifor Pharma, Vifor Pharma, and Vifor Pharma, outside the submitted work; and led the development of the AAV PRO, GCA PRO and Steroid PRO. The authors report no other conflicts of interest in this work.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous