A Cu(ii)-MOF based on a propargyl carbamate-functionalized isophthalate ligand
- PMID: 35479884
- PMCID: PMC9034020
- DOI: 10.1039/d1ra02686k
A Cu(ii)-MOF based on a propargyl carbamate-functionalized isophthalate ligand
Abstract
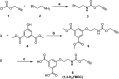
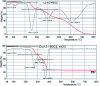
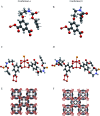
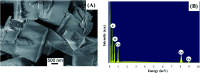
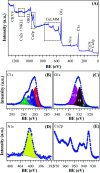
A copper-based metal-organic framework (MOF) was prepared using a new linker, a 5-substituted isophthalic acid bearing a propargyl carbamate group, intended to provide a terminal alkyne function protruding from the material surface to generate supported gold species for potential catalytic applications. The novel material was fully characterized by spectroscopic analyses of different kinds: FTIR, Raman, EDX, and XPS, as well as by thermal and surface area measurements. Synchrotron X-ray diffraction data analysis, in particular, revealed that this MOF, labelled [Cu(1,3-YBDC)]·xH2O (x ∼ 2), where Y stands for the pendant alkYne and BDC for benzene dicarboxylate, contains a complex network of 5-substituted isophthalate anions bound to Cu(ii) centers, arranged in pairs within paddlewheel (or "Chinese lantern") fragments of Cu2(μ-COO)4(D)2 formulation (D being a neutral Lewis base), with a short Cu⋯Cu distance of 2.633(4) Å. Quite unexpectedly, the apical atom in the paddlewheel structure belongs to the carbamate carbonyl oxygen atom. Such extra coordination by the propargyl carbamate groups drastically reduces the MOF porosity, a feature that was also confirmed by BET measurements. However, the MOF functionality is retained at the external crystal surface where 2% of active terminal alkynes is located.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
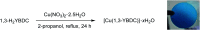
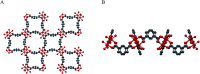

References
LinkOut - more resources
Full Text Sources
Research Materials

