Elucidation of molecular interactions of theaflavin monogallate with camel milk lactoferrin: detailed spectroscopic and dynamic simulation studies
- PMID: 35479994
- PMCID: PMC9037349
- DOI: 10.1039/d1ra03256a
Elucidation of molecular interactions of theaflavin monogallate with camel milk lactoferrin: detailed spectroscopic and dynamic simulation studies
Abstract
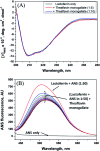
Lactoferrin is a heme-binding multifunctional glycoprotein known for iron transportation in the blood and also contributes to innate immunity. In this study, the interaction of theaflavin monogallate, a polyphenolic component of black tea, with camel milk lactoferrin was studied using various biophysical and computational techniques. Fluorescence quenching at different temperatures suggests that theaflavin monogallate interacted with lactoferrin by forming a non-fluorescent complex, i.e., static quenching. Theaflavin monogallate shows a significant affinity towards lactoferrin with a binding constant of ∼104-105 M-1 at different temperatures. ANS binding shows that the binding of polyphenol resulted in the burial of hydrophobic domains of lactoferrin. Moreover, thermodynamic parameters (ΔH, ΔS and ΔG) suggested that the interaction between protein and polyphenol was entropically favored and spontaneous. Circular dichroism confirmed there was no alteration in the secondary structure of lactoferrin. The energy transfer efficiency (FRET) from lactoferrin to theaflavin was found to be approximately 50%, with a distance between protein and polyphenol of 2.44 nm. Molecular docking shows that the binding energy of lactoferrin-theaflavin monogallate interaction was -9.7 kcal mol-1. Theaflavin monogallate was bound at the central cavity of lactoferrin and formed hydrogen bonds with Gln89, Tyr192, Lys301, Ser303, Gln87, and Val250 of lactoferrin. Other residues, such as Tyr82, Tyr92, and Tyr192, were involved in hydrophobic interactions. The calculation of various molecular dynamics simulations parameters indicated the formation of a stable complex between protein and polyphenol. This study delineates the binding mechanism of polyphenol with milk protein and could be helpful in milk formulations and play a key role in the food industry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Furlund C. B. Ulleberg E. K. Devold T. G. Flengsrud R. Jacobsen M. Sekse C. Holm H. Vegarud G. E. Identification of lactoferrin peptides generated by digestion with human gastrointestinal enzymes. J. Dairy Sci. 2013;96:75–88. doi: 10.3168/jds.2012-5946. doi: 10.3168/jds.2012-5946. - DOI - DOI - PubMed
-
- Cohen M. S. Mao J. Rasmussen G. T. Serody J. S. Britigan B. E. Interaction of Lactoferrin and Lipopolysaccharide (LPS): Effects on the Antioxidant Property of Lactoferrin and the Ability of LPS to Prime Human Neutrophils for Enhanced Superoxide Formation. J. Infect. Dis. 1992;166:1375. doi: 10.1093/infdis/166.6.1375. doi: 10.1093/infdis/166.6.1375. - DOI - DOI - PubMed
LinkOut - more resources
Full Text Sources

