Medicinal chemistry inspired by ginger: exploring the chemical space around 6-gingerol
- PMID: 35480015
- PMCID: PMC9037716
- DOI: 10.1039/d1ra04227k
Medicinal chemistry inspired by ginger: exploring the chemical space around 6-gingerol
Abstract
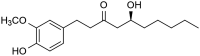
Ginger (Zingiber officinale Roscoe) has been used as a spice and as a traditional remedy since ancient times, especially in traditional Chinese medicine. It has been applied as a treatment for many diseases either alone or in combination with other remedies. Many studies were conducted on ginger and its constituents and a wide array of bioactivities were reported, e.g., antioxidant, anti-inflammatory, antiemetic, and anticancer activity. Most of these had been correlated to gingerols and shogaols, the most abundant secondary metabolites in ginger. This inspired several research groups to explore the biomedical value of the chemical space around these compounds, and many of their synthetic or semi-synthetic analogues have been prepared and studied for various bioactivities. Thanks to this, many valuable structure activity relationships have been revealed for such compounds. Herein, we provide a brief summary on the synthetic derivatization efforts that had so far been implemented on 6-gingerol, the main constituent of fresh ginger. This review covers 160 natural, semisynthetic, or synthetic 6-gingerol derivatives and their reported bioactivities.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


Similar articles
-
Immunomodulatory and anti-inflammatory therapeutic potential of gingerols and their nanoformulations.Front Pharmacol. 2022 Sep 5;13:902551. doi: 10.3389/fphar.2022.902551. eCollection 2022. Front Pharmacol. 2022. PMID: 36133811 Free PMC article. Review.
-
Characterization of gingerol analogues in supercritical carbon dioxide (SC CO2) extract of ginger (Zingiber officinale, R.,).J Food Sci Technol. 2014 Nov;51(11):3383-9. doi: 10.1007/s13197-012-0851-4. Epub 2012 Sep 23. J Food Sci Technol. 2014. PMID: 26396335 Free PMC article.
-
Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe).Foods. 2019 May 30;8(6):185. doi: 10.3390/foods8060185. Foods. 2019. PMID: 31151279 Free PMC article. Review.
-
Effect of drying and processing on diterpenes and other chemical constituents of ginger.J Nat Med. 2023 Jan;77(1):118-127. doi: 10.1007/s11418-022-01652-z. Epub 2022 Oct 9. J Nat Med. 2023. PMID: 36209453
-
Bioactivities and green advanced extraction technologies of ginger oleoresin extracts: A review.Food Res Int. 2023 Feb;164:112283. doi: 10.1016/j.foodres.2022.112283. Epub 2022 Dec 5. Food Res Int. 2023. PMID: 36737895 Review.
Cited by
-
Development of raft-forming liquid formulations loaded with ginger extract-solid dispersion for treatment of gastric ulceration.Heliyon. 2024 May 23;10(11):e31803. doi: 10.1016/j.heliyon.2024.e31803. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38841494 Free PMC article.
-
Investigation of the Zingerone's effects on wound healing in induced diabetic rats model.Arch Dermatol Res. 2025 Feb 24;317(1):484. doi: 10.1007/s00403-025-03924-6. Arch Dermatol Res. 2025. PMID: 39994064
-
Histone Deacetylase Inhibitory Activity and Antiproliferative Potential of New [6]-Shogaol Derivatives.Molecules. 2022 May 22;27(10):3332. doi: 10.3390/molecules27103332. Molecules. 2022. PMID: 35630809 Free PMC article.
-
Preparation and Evaluation of 6-Gingerol Derivatives as Novel Antioxidants and Antiplatelet Agents.Antioxidants (Basel). 2023 Mar 17;12(3):744. doi: 10.3390/antiox12030744. Antioxidants (Basel). 2023. PMID: 36978992 Free PMC article.
-
Polyphenolic Compounds Activate SERCA1a and Attenuate Methylglyoxal- and Palmitate-Induced Impairment in Pancreatic INS-1E Beta Cells.Cells. 2024 Nov 9;13(22):1860. doi: 10.3390/cells13221860. Cells. 2024. PMID: 39594609 Free PMC article.
References
-
- Nile S. H. Se Won P. Ind. Crops Prod. 2015;70:238–244. doi: 10.1016/j.indcrop.2015.03.033. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

