A Critical Review and Meta-Analysis of Impacts of Per- and Polyfluorinated Substances on the Brain and Behavior
- PMID: 35480070
- PMCID: PMC9035516
- DOI: 10.3389/ftox.2022.881584
A Critical Review and Meta-Analysis of Impacts of Per- and Polyfluorinated Substances on the Brain and Behavior
Abstract
Per- and polyfluoroalkyl substances (PFAS) are a class of structurally diverse synthetic organic chemicals that are chemically stable, resistant to degradation, and persistent in terrestrial and aquatic environments. Widespread use of PFAS in industrial processing and manufacturing over the last 70 years has led to global contamination of built and natural environments. The brain is a lipid rich and highly vascularized organ composed of long-lived neurons and glial cells that are especially vulnerable to the impacts of persistent and lipophilic toxicants. Generally, PFAS partition to protein-rich tissues of the body, primarily the liver and blood, but are also detected in the brains of humans, wildlife, and laboratory animals. Here we review factors impacting the absorption, distribution, and accumulation of PFAS in the brain, and currently available evidence for neurotoxic impacts defined by disruption of neurochemical, neurophysiological, and behavioral endpoints. Emphasis is placed on the neurotoxic potential of exposures during critical periods of development and in sensitive populations, and factors that may exacerbate neurotoxicity of PFAS. While limitations and inconsistencies across studies exist, the available body of evidence suggests that the neurobehavioral impacts of long-chain PFAS exposures during development are more pronounced than impacts resulting from exposure during adulthood. There is a paucity of experimental studies evaluating neurobehavioral and molecular mechanisms of short-chain PFAS, and even greater data gaps in the analysis of neurotoxicity for PFAS outside of the perfluoroalkyl acids. Whereas most experimental studies were focused on acute and subchronic impacts resulting from high dose exposures to a single PFAS congener, more realistic exposures for humans and wildlife are mixtures exposures that are relatively chronic and low dose in nature. Our evaluation of the available human epidemiological, experimental, and wildlife data also indicates heightened accumulation of perfluoroalkyl acids in the brain after environmental exposure, in comparison to the experimental studies. These findings highlight the need for additional experimental analysis of neurodevelopmental impacts of environmentally relevant concentrations and complex mixtures of PFAS.
Keywords: PFAS; behavior; blood-brain barrier; brain; development; fluorochemical; neurotoxicity; persistent.
Copyright © 2022 Starnes, Rock, Jackson and Belcher.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
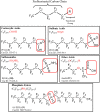

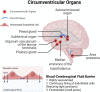
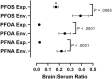
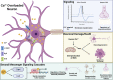


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

