Preparation of quinazolinones using biosynthesized silver nanoparticles
- PMID: 35480348
- PMCID: PMC9036550
- DOI: 10.1039/d2ra01629j
Preparation of quinazolinones using biosynthesized silver nanoparticles
Abstract
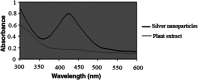
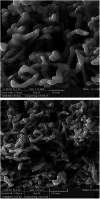
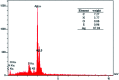
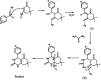
A silver nanocatalyst has been used as an effective catalyst for the preparation of quinazolinones under reflux conditions in ethanol. The catalyst was characterized by UV-VIS, FT-IR, XRD, SEM and EDS. Amongst the many benefits of this method are atom economy, reusability of the catalyst, low catalyst loading, applicability to a wide range of substrates, high yields of products, environmental friendliness and easy separation of products. Silver nanoparticles (Ag NPs) were prepared using Echium amoenum extract. The structures of the prepared quinazolinones were fully characterized by 1H and 13C NMR, FT-IR spectra and elemental analysis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Al-Amiery A. A. Kadhum A. A. H. Shamel M. Satar M. Khalid Y. Mohamad A. B. Med. Chem. Res. 2014;23:236–242. doi: 10.1007/s00044-013-0625-1. - DOI
LinkOut - more resources
Full Text Sources

