Synthesis of 2-methoxybenzamide derivatives and evaluation of their hedgehog signaling pathway inhibition
- PMID: 35480433
- PMCID: PMC9034380
- DOI: 10.1039/d1ra00732g
Synthesis of 2-methoxybenzamide derivatives and evaluation of their hedgehog signaling pathway inhibition
Abstract
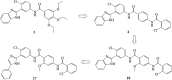
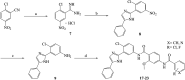
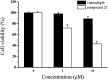
Aberrant hedgehog (Hh) signaling is implicated in the development of a variety of cancers. Smoothened (Smo) protein is a bottleneck in the Hh signal transduction. The regulation of the Hh signaling pathway to target the Smo receptor is a practical approach for development of anticancer agents. We report herein the design and synthesis of a series of 2-methoxybenzamide derivatives as Hh signaling pathway inhibitors. The pharmacological data demonstrated that compound 21 possessed potent Hh pathway inhibition with a nanomolar IC50 value, and it prevented Shh-induced Smo from entering the primary cilium. Furthermore, mutant Smo was effectively suppressed via compound 21. The in vitro antiproliferative activity of compound 21 against a drug-resistant cell line gave encouraging results.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
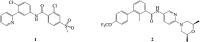

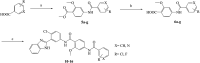



References
LinkOut - more resources
Full Text Sources
Miscellaneous

