Comparative transmissibility of SARS-CoV-2 variants Delta and Alpha in New England, USA
- PMID: 35480627
- PMCID: PMC8913280
- DOI: 10.1016/j.xcrm.2022.100583
Comparative transmissibility of SARS-CoV-2 variants Delta and Alpha in New England, USA
Abstract
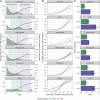
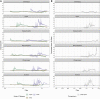
The SARS-CoV-2 Delta variant rose to dominance in mid-2021, likely propelled by an estimated 40%-80% increased transmissibility over Alpha. To investigate if this ostensible difference in transmissibility is uniform across populations, we partner with public health programs from all six states in New England in the United States. We compare logistic growth rates during each variant's respective emergence period, finding that Delta emerged 1.37-2.63 times faster than Alpha (range across states). We compute variant-specific effective reproductive numbers, estimating that Delta is 63%-167% more transmissible than Alpha (range across states). Finally, we estimate that Delta infections generate on average 6.2 (95% CI 3.1-10.9) times more viral RNA copies per milliliter than Alpha infections during their respective emergence. Overall, our evidence suggests that Delta's enhanced transmissibility can be attributed to its innate ability to increase infectiousness, but its epidemiological dynamics may vary depending on underlying population attributes and sequencing data availability.
Keywords: COVID-19; SARS-CoV-2; VOC; genomic epidemiology; transmissibility; variant of concern; viral emergence; viral sequencing.
© 2022 The Authors.
Conflict of interest statement
N.D.G. is a paid consultant for Tempus Labs and the National Basketball Association and has received speaking fees from Goldman Sachs. P.C.S. is a co-founder of, shareholder in, and scientific advisor to Sherlock Biosciences, Inc., as well as a board member of and shareholder in Danaher Corporation. The remaining authors declare no competing interests.
Figures



Update of
-
Comparative transmissibility of SARS-CoV-2 variants Delta and Alpha in New England, USA.medRxiv [Preprint]. 2021 Oct 7:2021.10.06.21264641. doi: 10.1101/2021.10.06.21264641. medRxiv. 2021. Update in: Cell Rep Med. 2022 Mar 11;3(4):100583. doi: 10.1016/j.xcrm.2022.100583. PMID: 34642698 Free PMC article. Updated. Preprint.
References
-
- CDC SARS-CoV-2 variant classifications and definitions. 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html
-
- Lauring A.S., Hodcroft E.B. Genetic variants of SARS-CoV-2—what do they mean? JAMA. 2021;325:529–531. - PubMed
-
- Rambaut A., Loman N., Pybus O., Barclay W., Barrett J., Carabelli A., Connor T., Peacock T., Robertson D.L., Volz E., et al. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations. 2020. https://virological.org/t/preliminary-genomic-characterisation-of-an-eme...
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

