Baseline Mutations and Up-Regulation of PI3K-AKT Pathway Serve as Potential Indicators of Lack of Response to Neoadjuvant Chemotherapy in Stage II/III Breast Cancer
- PMID: 35480699
- PMCID: PMC9036956
- DOI: 10.3389/fonc.2021.784985
Baseline Mutations and Up-Regulation of PI3K-AKT Pathway Serve as Potential Indicators of Lack of Response to Neoadjuvant Chemotherapy in Stage II/III Breast Cancer
Abstract
Background: Neoadjuvant chemotherapy (NAC) has been expanded to hormone receptor (HR) positive breast cancer (BC) patients with operable disease, to increase the likelihood of breast-conserving surgery. Genomic profiling at baseline would reveal NAC response relevant genomic features and signaling pathways, guiding clinical NAC utilization based on patients' genomic characteristics.
Methods: We prospectively studied stage II/III BC patients who were eligible for breast-conserving surgery. Patients received epirubicin and cyclophosphamide for 4 cycles, followed by another 4-cycle docetaxel, and human epidermal growth factor receptor (HER2) positive patients were additionally treated with herceptin when using docetaxel (EC-T(H)). NAC responses were evaluated as pathologic complete response (pCR) or non-pathologic complete response (non-pCR). Genomic features related to NAC responses were identified by profiling baseline tumor tissues sampled one day before NAC, using whole-exome sequencing. Differentially expressed genes and up-/down-regulated pathways were investigated by performing RNA-sequencing.
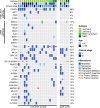
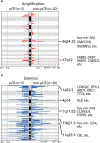
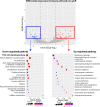
Results: A total of 25 stage II/III BC patients were enrolled, including 5 patients ultimately evaluated as pCR and 20 patients evaluated as non-pCR. PIK3CA (48%) and TP53 (40%) mutations were enriched in patients not achieving pCR. Mutated phosphatidylinositol-3-kinase-AKT (PI3K-AKT) pathway and homologous recombinational repair pathway were also more frequently observed in patients evaluated as non-pCR. Significant arm-level amplifications (8q24.23 and 17q12) and deletions (1p32.2, 4p14, 7q11.23, 10q21.3, 11q23.3, etc.) were identified among patients not achieving pCR, while patients achieving pCR displayed no significant copy number alterations. Significantly up-regulated expression of PI3K-AKT pathway genes was also detected among patients failed to achieve pCR, compared to patients achieving pCR.
Conclusion: Compared to BC patients achieving pCR to NAC, aberrant activation of PI3K-AKT pathway genes were more frequently observed in patients not achieving pCR, consistent with the significant up-regulation of PI3K-AKT pathway gene expression in the non-pCR subgroup. Together, these findings indicate that upregulated PI3K-AKT pathway serves as a potential indicator of lack of response to NAC in stage II/III BC patients, and other effective therapeutic options are urgently needed for those resistant patients.
Keywords: PI3K-AKT pathway; PIK3CA mutations; neoadjuvant chemotherapy (NAC); pathologic complete response (pCR); stage II/III breast cancer (BC).
Copyright © 2022 Dong, Shan, Han, Zhao, Wang, Zhu, Ou, Ma and Pan.
Conflict of interest statement
XZ, FW, LZ, and QO are employees of Nanjing Geneseeq Technology Inc., China. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Impact of somatic PI3K pathway and ERBB family mutations on pathological complete response (pCR) in HER2-positive breast cancer patients who received neoadjuvant HER2-targeted therapies.Breast Cancer Res. 2017 Jul 27;19(1):87. doi: 10.1186/s13058-017-0883-9. Breast Cancer Res. 2017. PMID: 28750640 Free PMC article. Clinical Trial.
-
Unraveling breast cancer response to neoadjuvant chemotherapy through integrated genomic, transcriptomic, and circulating tumor DNA analysis.Breast Cancer Res. 2025 May 1;27(1):64. doi: 10.1186/s13058-025-02026-5. Breast Cancer Res. 2025. PMID: 40312292 Free PMC article.
-
Long-term outcome of the REMAGUS 02 trial, a multicenter randomised phase II trial in locally advanced breast cancer patients treated with neoadjuvant chemotherapy with or without celecoxib or trastuzumab according to HER2 status.Eur J Cancer. 2017 Apr;75:323-332. doi: 10.1016/j.ejca.2017.01.008. Epub 2017 Mar 7. Eur J Cancer. 2017. PMID: 28279941 Clinical Trial.
-
A nomogram to predict pathologic complete response (pCR) and the value of tumor-infiltrating lymphocytes (TILs) for prediction of response to neoadjuvant chemotherapy (NAC) in breast cancer patients.Breast Cancer Res Treat. 2019 Jan;173(2):255-266. doi: 10.1007/s10549-018-4981-x. Epub 2018 Oct 15. Breast Cancer Res Treat. 2019. PMID: 30324273 Review.
-
Complete pathologic response rate to neoadjuvant chemotherapy increases with increasing HER2/CEP17 ratio in HER2 overexpressing breast cancer: analysis of the National Cancer Database (NCDB).Breast Cancer Res Treat. 2020 Jun;181(2):249-254. doi: 10.1007/s10549-020-05599-1. Epub 2020 Apr 10. Breast Cancer Res Treat. 2020. PMID: 32277375 Review.
Cited by
-
Anti-tumor effects of low-dose metronomic vinorelbine in combination with alpelisib in breast cancer cells.EXCLI J. 2023 Jan 13;22:114-130. doi: 10.17179/excli2022-5064. eCollection 2023. EXCLI J. 2023. PMID: 36998707 Free PMC article.
-
Whole-exome profiles of inflammatory breast cancer and pathological response to neoadjuvant chemotherapy.J Transl Med. 2024 Oct 27;22(1):969. doi: 10.1186/s12967-024-05790-8. J Transl Med. 2024. PMID: 39465437 Free PMC article.
-
Downregulation of RORl via STAT3 and P300 Promotes P38 Pathway- Dependent Lens Epithelial Cells Apoptosis in Age-Related Cataract.Biochem Genet. 2025 Feb 28. doi: 10.1007/s10528-025-11067-6. Online ahead of print. Biochem Genet. 2025. PMID: 40019609
-
Pathologic Response and Survival Outcomes on HER2-Low vs. HER2-Zero in Breast Cancer Receiving Neoadjuvant Chemotherapy.Medicina (Kaunas). 2025 Jun 27;61(7):1168. doi: 10.3390/medicina61071168. Medicina (Kaunas). 2025. PMID: 40731799 Free PMC article.
-
Systematic review: genetic polymorphisms in the pharmacokinetics of high-dose methotrexate in pediatric acute lymphoblastic leukemia patients.Cancer Chemother Pharmacol. 2024 Aug;94(2):141-155. doi: 10.1007/s00280-024-04694-0. Epub 2024 Jul 13. Cancer Chemother Pharmacol. 2024. PMID: 39002021
References
-
- Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. . Effect of Preoperative Chemotherapy on Local-Regional Disease in Women With Operable Breast Cancer: Findings From National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol (1997) 15(7):2483–93. doi: 10.1200/JCO.1997.15.7.2483 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

