Sensing mechanism of a new fluorescent probe for hydrogen sulfide: photoinduced electron transfer and invalidity of excited-state intramolecular proton transfer
- PMID: 35480821
- PMCID: PMC9034181
- DOI: 10.1039/d1ra02511b
Sensing mechanism of a new fluorescent probe for hydrogen sulfide: photoinduced electron transfer and invalidity of excited-state intramolecular proton transfer
Abstract
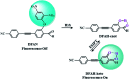
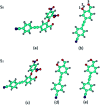
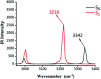
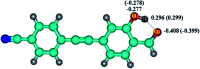
It is of great significance for biological research to develop efficient detection methods of hydrogen sulfide (H2S). When DFAN reacts with H2S, 2,4-dinitrophenyl ether group acting as an electron acceptor generates a hydroxyl-substituted 2,4-dinitrophenyl ether group, resulting in the disappearance of photoinduced electron transfer (PET), and the new formed DFAH can be observed, while being accompanied by a significant fluorescence. In the present study, the PET sensing mechanism of probe DFAN and the excited state intramolecular proton transfer (ESIPT) process of DFAH have been explored in detail based on the density functional theory (DFT) and time-dependent density functional theory (TD-DFT) methods. Our theoretical results show that the fluorescence quenching of DFAN is caused by the PET mechanism, and the result of ESIPT mechanism is not due to the large Stokes shift fluorescence emission of DFAH. We also optimized the geometric structure of the transition state of DFAH. The frontier molecular orbitals and potential barrier show that the ESIPT process does not easy occur easily for DFAH. The enol structure of DFAH is more stable than that of the keto structure. The absence of the PET process resulted in the enol structure emitting strong fluorescence, which is consistent with the single fluorescence in the experiment. Above all, our calculations are sufficient to verify the sensing mechanism of H2S using DFAN.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
LinkOut - more resources
Full Text Sources

