Protein detection enabled using functionalised silk-binding peptides on a silk-coated optical fibre
- PMID: 35480827
- PMCID: PMC9034238
- DOI: 10.1039/d1ra03584c
Protein detection enabled using functionalised silk-binding peptides on a silk-coated optical fibre
Abstract
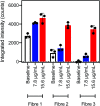
We present a new coating procedure to prepare optical fibre sensors suitable for use with protein analytes. We demonstrate this through the detection of AlexaFluor-532 tagged streptavidin by its binding to D-biotin that is functionalised onto an optical fibre, via incorporation in a silk fibroin fibre coating. The D-biotin was covalently attached to a silk-binding peptide to provide SBP-biotin, which adheres the D-biotin to the silk-coated fibre tip. These optical fibre probes were prepared by two methods. The first involves dip-coating the fibre tip into a mixture of silk fibroin and SBP-biotin, which distributes the SBP-biotin throughout the silk coating (method A). The second method uses two steps, where the fibre is first dip-coated in silk only, then SBP-biotin added in a second dip-coating step. This isolates SBP-biotin to the outer surface of the silk layer (method B). A series of fluorescence measurements revealed that only the surface bound SBP-biotin detects streptavidin with a detection limit of 15 μg mL-1. The fibre coatings are stable to repeated washing and long-term exposure to water. Formation of silk coatings on fibres using commercial aqueous silk fibroin was found to be inhibited by a lithium concentration of 200 ppm, as determined by atomic absorption spectroscopy. This was reduced to less than 20 ppm by dialysis against water, and was found to successfully form a coating on optical fibres.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
RAM is a co-founder and a Director of Miniprobes Pty Ltd, a company that develops novel optical imaging systems. Miniprobes Pty Ltd did not contribute to this study.
Figures



Similar articles
-
Glycomonoterpene-Functionalized Crack-Resistant Biocompatible Silk Fibroin Coatings for Biomedical Implants.ACS Appl Bio Mater. 2019 Feb 18;2(2):675-684. doi: 10.1021/acsabm.8b00515. Epub 2019 Jan 31. ACS Appl Bio Mater. 2019. PMID: 35016273
-
Development of antibiotic-loaded silk fibroin/hyaluronic acid polyelectrolyte film coated CoCrMo alloy.Biomed Tech (Berl). 2016 Oct 1;61(5):463-474. doi: 10.1515/bmt-2015-0061. Biomed Tech (Berl). 2016. PMID: 26540212
-
Effects of pH on the silk fibroin coating of CoCrMo alloy.Scanning. 2016 Jan-Feb;38(1):80-8. doi: 10.1002/sca.21224. Epub 2015 May 13. Scanning. 2016. PMID: 25970826
-
Silk fibroin film-coated MgZnCa alloy with enhanced in vitro and in vivo performance prepared using surface activation.Acta Biomater. 2019 Jun;91:99-111. doi: 10.1016/j.actbio.2019.04.048. Epub 2019 Apr 24. Acta Biomater. 2019. PMID: 31028907
-
G measurements with time-of-swing method at HUST.Philos Trans A Math Phys Eng Sci. 2014 Oct 13;372(2026):20140141. doi: 10.1098/rsta.2014.0141. Epub 2014 Sep 8. Philos Trans A Math Phys Eng Sci. 2014. PMID: 25202004 Free PMC article. Review.
Cited by
-
Single-fiber probes for combined sensing and imaging in biological tissue: recent developments and prospects.Biomed Opt Express. 2024 Mar 15;15(4):2392-2405. doi: 10.1364/BOE.517920. eCollection 2024 Apr 1. Biomed Opt Express. 2024. PMID: 38633092 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

