Nanodelivery of nucleic acids
- PMID: 35480987
- PMCID: PMC9038125
- DOI: 10.1038/s43586-022-00104-y
Nanodelivery of nucleic acids
Abstract
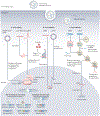
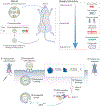
There is growing need for a safe, efficient, specific and non-pathogenic means for delivery of gene therapy materials. Nanomaterials for nucleic acid delivery offer an unprecedented opportunity to overcome these drawbacks; owing to their tunability with diverse physico-chemical properties, they can readily be functionalized with any type of biomolecules/moieties for selective targeting. Nucleic acid therapeutics such as antisense DNA, mRNA, small interfering RNA (siRNA) or microRNA (miRNA) have been widely explored to modulate DNA or RNA expression Strikingly, gene therapies combined with nanoscale delivery systems have broadened the therapeutic and biomedical applications of these molecules, such as bioanalysis, gene silencing, protein replacement and vaccines. Here, we overview how to design smart nucleic acid delivery methods, which provide functionality and efficacy in the layout of molecular diagnostics and therapeutic systems. It is crucial to outline some of the general design considerations of nucleic acid delivery nanoparticles, their extraordinary properties and the structure-function relationships of these nanomaterials with biological systems and diseased cells and tissues.
Conflict of interest statement
Competing interests J. Conde is a co-founder and shareholder of TargTex S.A. The other authors declare no competing interests.
Figures





References
-
- Global Burden of Disease 2019 Cancer Collaboration. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. JAMA Oncol . 10.1001/jamaoncol.2021.6987 (2021). - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
