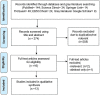
A systematic review of pharmaceutical price mark-up practice and its implementation
- PMID: 35481119
- PMCID: PMC9031039
- DOI: 10.1016/j.rcsop.2021.100020
A systematic review of pharmaceutical price mark-up practice and its implementation
Abstract
Pharmaceutical products, apart from being essential for medical treatment, are of high value and heavily regulated to ensure the prices are controlled. This systematic review was conducted to identify pharmaceutical pricing mark-up control measures, specifically in the wholesale and retail sectors. The search method comprised the following databases: PubMed, Science Direct, Springer Link, ProQuest, and EBSCOhost and Google Scholar. The results were filtered systematically from the inception of the aforementioned databases until 23 April 2021. Eligible studies were those focusing on the implementation of pharmaceutical pricing strategies that involve a) mark-ups of medicine, and b) pharmaceutical cost control measures. A total of 13 studies were included in this review: seven covered European countries, four covered Asian countries, one covered the USA and one covered Canada. The main points of discussion in the qualitative synthesis were the implementation of medicine mark-ups, price mark-up regulatory strategies and the outcomes of these regulatory strategies. Our findings suggest that Western countries have a lower mark-up margin, around 4% to 25% of the original purchased price, compared to Asian countries, up to 50%.
Keywords: Cost control; Drug costs; Healthcare costs; Pharmaceutical economics; Prescription fees; Price list.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Determinants of drug prices: a systematic review of comparison studies.BMJ Open. 2021 Jul 15;11(7):e046917. doi: 10.1136/bmjopen-2020-046917. BMJ Open. 2021. PMID: 34266841 Free PMC article.
-
Factors Impacting Pharmaceutical Prices and Affordability: Narrative Review.Pharmacy (Basel). 2020 Dec 23;9(1):1. doi: 10.3390/pharmacy9010001. Pharmacy (Basel). 2020. PMID: 33374493 Free PMC article. Review.
-
A systematic review of policies regulating or removing mark-ups in the pharmaceutical supply and distribution chain.Health Policy. 2023 Dec;138:104919. doi: 10.1016/j.healthpol.2023.104919. Epub 2023 Sep 28. Health Policy. 2023. PMID: 37788559
-
Impact of European pharmaceutical price regulation on generic price competition: a review.Pharmacoeconomics. 2010;28(8):649-63. doi: 10.2165/11535360-000000000-00000. Pharmacoeconomics. 2010. PMID: 20515079 Review.
-
Association between medicine Price declaration by pharmaceutical industries and retail prices in Malaysia's private healthcare sector.J Pharm Policy Pract. 2019 Jul 3;12:15. doi: 10.1186/s40545-019-0176-z. eCollection 2019. J Pharm Policy Pract. 2019. PMID: 31304021 Free PMC article.
Cited by
-
Regulation of mark-up on medicine prices in Zimbabwe: a pilot survey from 92 community pharmacies in the metropolitan area of Harare.Health Econ Rev. 2024 Nov 15;14(1):92. doi: 10.1186/s13561-024-00574-8. Health Econ Rev. 2024. PMID: 39546124 Free PMC article.
-
Exploring the effectiveness of demand-side retail pharmaceutical expenditure reforms : Cross-country evidence from weighted-average least squares estimation.Int J Health Econ Manag. 2023 Mar;23(1):149-172. doi: 10.1007/s10754-022-09337-6. Epub 2022 Sep 21. Int J Health Econ Manag. 2023. PMID: 36131191 Free PMC article.
-
Replenish Revenue by Low-Cost Medicines, an Institution-Specific Action to Improve Access to High-Cost Medicines Used for Chronic Illness in Ethiopia: Narrative Review.Health Sci Rep. 2025 Apr 29;8(5):e70775. doi: 10.1002/hsr2.70775. eCollection 2025 May. Health Sci Rep. 2025. PMID: 40309621 Free PMC article.
-
The Access to Oncology Medicines Coalition: Enhanced in-Country Coordination for Sustainable Access.Cancer Manag Res. 2024 Nov 16;16:1609-1616. doi: 10.2147/CMAR.S459873. eCollection 2024. Cancer Manag Res. 2024. PMID: 39575163 Free PMC article.
-
An assessment of the implications of distribution remuneration and taxation policies on the final prices of prescription medicines: evidence from 35 countries.Eur J Health Econ. 2025 Apr;26(3):513-536. doi: 10.1007/s10198-024-01706-x. Epub 2024 Sep 19. Eur J Health Econ. 2025. PMID: 39299943 Free PMC article.
References
-
- Espin J., Rovira J., Labry A.O.D. Review Series on Pharmaceutical Pricing Policies and Interventions 2011. 2011. Who/hai project on medicine prices and availability. Working Paper 1: External Reference Pricing.
-
- Ondo Z. Regulatory analysis of mark-up structure in medicine prices by the pharmaceutical industry in south africa. J Pharm Care Health Syst. 2019;6(2):203. doi: 10.35248/2376-0419.19.6.203. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous