Simple manipulation of enzyme-linked immunosorbent assay (ELISA) using an automated microfluidic interface
- PMID: 35481474
- PMCID: PMC9119197
- DOI: 10.1039/d2ay00326k
Simple manipulation of enzyme-linked immunosorbent assay (ELISA) using an automated microfluidic interface
Abstract
Among lateral flow immunoassay (LFIA) platforms, enzyme-based LFIAs provide signal amplification to improve sensitivity. However, most enzyme-based LFIAs require multiple timed steps, complicating their utility in point-of-care testing (POCT). Here, we report a microfluidic interface for LFIAs that automates sample, buffer, and reagent addition, greatly simplifying operation while achieving the high analytical stringency associated with more complex assays. The microfluidic interface also maintains the low cost and small footprint of standard LFIAs. The platform is fabricated from a combination of polyester film, double-sided adhesive tape, and nitrocellulose, and fits in the palm of your hand. All reagents are dried on the nitrocellulose to facilitate sequential reagent delivery, and the sample is used as the wash buffer to minimize steps. After the sample addition, a user simply waits 15 min for a colorimetric result. This manuscript discusses the development and optimization of the channel geometry to achieve a simple step enzyme amplified immunoassay. As a proof-of-concept target, we selected lipoarabinomannan (LAM), a WHO identified urinary biomarker of active tuberculosis, to demonstrate the device feasibility and reliability. The results revealed that the device successfully detected LAM in phosphate buffer (PBS) as well as spiked urine samples within 15 min after sample loading. The minimum concentration of color change was achieved at 25 ng mL-1.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures
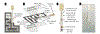
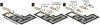



References
-
- Lai S, Wang S, Luo J, Lee LJ, Yang S-T and Madou MJ, Analytical Chemistry, 2004, 76, 1832–1837. - PubMed
-
- Zhang Z, Chen Z, Wang S, Cheng F and Chen L, ACS Applied Materials & Interfaces, 2015, 7, 27639–27645. - PubMed
-
- Li Y, Ma X, Xu Z, Liu M, Lin Z, Qiu B, Guo L and Chen G, Analyst, 2016, 141, 2970–2976. - PubMed
-
- Posthuma-Trumpie GA, Korf J and van Amerongen A, Analytical and Bioanalytical Chemistry, 2009, 393, 569–582. - PubMed
-
- Kolosova AY, de Saeger S, Sibanda L, Verheijen R and van Peteghem C, Analytical and Bioanalytical Chemistry, 2007, 389, 2103–2107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

