Functionalization of a symmetric protein scaffold: Redundant folding nuclei and alternative oligomeric folding pathways
- PMID: 35481645
- PMCID: PMC8996475
- DOI: 10.1002/pro.4301
Functionalization of a symmetric protein scaffold: Redundant folding nuclei and alternative oligomeric folding pathways
Abstract
Successful de novo protein design ideally targets specific folding kinetics, stability thermodynamics, and biochemical functionality, and the simultaneous achievement of all these criteria in a single step design is challenging. Protein design is potentially simplified by separating the problem into two steps: (a) an initial design of a protein "scaffold" having appropriate folding kinetics and stability thermodynamics, followed by (b) appropriate functional mutation-possibly involving insertion of a peptide functional "cassette." This stepwise approach can also separate the orthogonal effects of the "stability/function" and "foldability/function" tradeoffs commonly observed in protein design. If the scaffold is a protein architecture having an exact rotational symmetry, then there is the potential for redundant folding nuclei and multiple equivalent sites of functionalization; thereby enabling broader functional adaptation. We describe such a "scaffold" and functional "cassette" design strategy applied to a β-trefoil threefold symmetric architecture and a heparin ligand functionality. The results support the availability of redundant folding nuclei within this symmetric architecture, and also identify a minimal peptide cassette conferring heparin affinity. The results also identify an energy barrier of destabilization that switches the protein folding pathway from monomeric to trimeric, thereby identifying another potential advantage of symmetric protein architecture in de novo design.
Keywords: de novo design; heparin affinity; oligomerization; protein folding; protein stability.
© 2022 The Protein Society.
Conflict of interest statement
Michael Blaber is a cofounder and has equity ownership in Trefoil Therapeutics Inc.
Figures


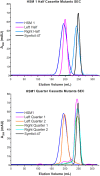
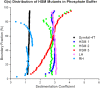
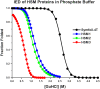




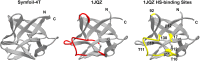
References
-
- Zhou W, Šmidlehner T, Jerala R. Synthetic biology principles for the design of protein with novel structures and functions. FEBS Lett. 2020;594:2199–2212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

