Molecularly imprinted materials for glycan recognition and processing
- PMID: 35481837
- PMCID: PMC9476894
- DOI: 10.1039/d2tb00164k
Molecularly imprinted materials for glycan recognition and processing
Abstract
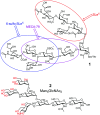
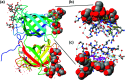
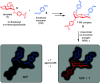
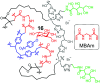
Carbohydrates are the most abundant organic molecules on Earth and glycosylation is the most common posttranslational modification of proteins. Glycans are involved in a plethora of biological processes including cell adhesion, bacterial and viral infection, inflammation, and cancer development. Coincidently, glycosides were some of the earliest molecules imprinted and have been instrumental in the development of covalent molecular imprinting technology. This perspective illustrates recently developed molecularly imprinted materials for glycan binding and processing. Novel imprinting techniques and postmodification led to development of synthetic glycan-binding materials capable of competing with natural lectins in affinity and artificial glycosidases for selective hydrolysis of complex glycans. These materials are expected to significantly advance glycochemistry, glycobiology, and related areas such as biomass conversion.
Conflict of interest statement
There are no conflicts to declare.
Figures







Similar articles
-
Boronate-Affinity Glycan-Oriented Surface Imprinting: A New Strategy to Mimic Lectins for the Recognition of an Intact Glycoprotein and Its Characteristic Fragments.Angew Chem Int Ed Engl. 2015 Aug 24;54(35):10211-5. doi: 10.1002/anie.201503066. Epub 2015 Jul 15. Angew Chem Int Ed Engl. 2015. PMID: 26179149
-
Precision Imprinting of Glycopeptides for Facile Preparation of Glycan-Specific Artificial Antibodies.Anal Chem. 2018 Aug 21;90(16):9845-9852. doi: 10.1021/acs.analchem.8b01903. Epub 2018 Aug 2. Anal Chem. 2018. PMID: 30036038
-
Glycan-specific molecularly imprinted polymers towards cancer diagnostics: merits, applications, and future perspectives.Chem Soc Rev. 2024 Feb 19;53(4):1870-1891. doi: 10.1039/d3cs00842h. Chem Soc Rev. 2024. PMID: 38223993 Review.
-
Selective Binding of Complex Glycans and Glycoproteins in Water by Molecularly Imprinted Nanoparticles.Nano Lett. 2020 Jul 8;20(7):5106-5110. doi: 10.1021/acs.nanolett.0c01305. Epub 2020 Jun 5. Nano Lett. 2020. PMID: 32501718 Free PMC article.
-
Advances in glycan-specific biomimetic molecular recognition and its biomedical applications.Chem Commun (Camb). 2025 May 1;61(37):6739-6754. doi: 10.1039/d5cc01003a. Chem Commun (Camb). 2025. PMID: 40243224 Review.
References
-
- N. R. Council, Transforming glycoscience: a roadmap for the future, National Academies Press, Washington, D.C., 2012 - PubMed
-
- Kamerling J. P. and Boons G.-J., Comprehensive glycoscience: from chemistry to systems biology, Elsevier, Amsterdam, Boston, 1st edn, 2007
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

