Regulation of 1 and 24 hydroxylation of vitamin D metabolites in the proximal tubule
- PMID: 35482362
- PMCID: PMC9335508
- DOI: 10.1177/15353702221091982
Regulation of 1 and 24 hydroxylation of vitamin D metabolites in the proximal tubule
Abstract
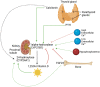
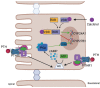
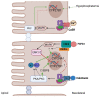
Calcium and phosphate are critical for numerous physiological processes. Consequently, the plasma concentration of these ions are tightly regulated. Calcitriol, the active form of vitamin D, is a positive modulator of mineralization as well as calcium and phosphate metabolism. The molecular and physiological effects of calcitriol are well documented. Calcitriol increases blood calcium and phosphate levels by increasing absorption from the intestine, and resorption of bone. Calcitriol synthesis is a multistep process. A precursor is first made via skin exposure to UV, it is then 25-hydroxylated in the liver to form 25-hydroxyitamin D. The next hydroxylation step occurs in the renal proximal tubule via the 1-αhydroxylase enzyme (encoded by CYP27B1) thereby generating 1,25-dihydroxyvitamin D, that is, calcitriol. At the same site, the 25-hydroxyvitamin D 24-hydroxlase enzyme encoded by CYP24A1 can hydroxylate 25-hydroxyvitamin D or calcitriol to deactivate the hormone. Plasma calcitriol levels are primarily determined by the regulated expression of CYP27B1 and CYP24A1. This occurs in response to parathyroid hormone (increases CYP27B1), calcitriol itself (decreases CYP27B1 and increases CYP24A1), calcitonin (increases or decreases CYP24A1 and increases CYP27B1), FGF23 (decreases CYP27B1 and increases CYP24A1) and potentially plasma calcium and phosphate levels themselves (mixed effects). Herein, we review the regulation of CYP27B1 and CYP24A1 transcription in response to the action of classic phophocalciotropic hormones and explore the possibility of direct regulation by plasma calcium.
Keywords: CYP24A1; CYP27B1; CaSR; PTH; Vitamin D; calcitriol; calcium; kidney.
Conflict of interest statement
Figures
Similar articles
-
Mechanistic homeostasis of vitamin D metabolism in the kidney through reciprocal modulation of Cyp27b1 and Cyp24a1 expression.J Steroid Biochem Mol Biol. 2020 Feb;196:105500. doi: 10.1016/j.jsbmb.2019.105500. Epub 2019 Oct 16. J Steroid Biochem Mol Biol. 2020. PMID: 31629064 Free PMC article. Review.
-
Genomic mechanisms controlling renal vitamin D metabolism.J Steroid Biochem Mol Biol. 2023 Apr;228:106252. doi: 10.1016/j.jsbmb.2023.106252. Epub 2023 Jan 16. J Steroid Biochem Mol Biol. 2023. PMID: 36657729 Free PMC article.
-
Rapid genomic changes by mineralotropic hormones and kinase SIK inhibition drive coordinated renal Cyp27b1 and Cyp24a1 expression via CREB modules.J Biol Chem. 2022 Nov;298(11):102559. doi: 10.1016/j.jbc.2022.102559. Epub 2022 Sep 30. J Biol Chem. 2022. PMID: 36183832 Free PMC article.
-
Temporal changes in tissue 1α,25-dihydroxyvitamin D3, vitamin D receptor target genes, and calcium and PTH levels after 1,25(OH)2D3 treatment in mice.Am J Physiol Endocrinol Metab. 2013 May 1;304(9):E977-89. doi: 10.1152/ajpendo.00489.2012. Epub 2013 Mar 12. Am J Physiol Endocrinol Metab. 2013. PMID: 23482451
-
Vitamin D Activity and Metabolism in Bone.Curr Osteoporos Rep. 2017 Oct;15(5):443-449. doi: 10.1007/s11914-017-0394-8. Curr Osteoporos Rep. 2017. PMID: 28808890 Review.
Cited by
-
A parathyroid hormone/salt-inducible kinase signaling axis controls renal vitamin D activation and organismal calcium homeostasis.J Clin Invest. 2023 May 1;133(9):e163627. doi: 10.1172/JCI163627. J Clin Invest. 2023. PMID: 36862513 Free PMC article.
-
Vitamin D exerts endogenous control over TH2 cell fate and immune plasticity.iScience. 2025 Feb 26;28(4):112117. doi: 10.1016/j.isci.2025.112117. eCollection 2025 Apr 18. iScience. 2025. PMID: 40224021 Free PMC article.
-
Association between Taql polymorphism of vitamin D receptor gene and vertical growth of the mandible: A cross-sectional study.Korean J Orthod. 2023 Sep 25;53(5):336-342. doi: 10.4041/kjod23.129. Korean J Orthod. 2023. PMID: 37746779 Free PMC article.
-
Confronting the global obesity epidemic: investigating the role and underlying mechanisms of vitamin D in metabolic syndrome management.Front Nutr. 2024 Aug 9;11:1416344. doi: 10.3389/fnut.2024.1416344. eCollection 2024. Front Nutr. 2024. PMID: 39183985 Free PMC article. Review.
-
Development of a kidney microphysiological system hardware platform for microgravity studies.NPJ Microgravity. 2024 May 11;10(1):54. doi: 10.1038/s41526-024-00398-0. NPJ Microgravity. 2024. PMID: 38734683 Free PMC article.
References
-
- Renkema KY, Alexander RT, Bindels RJ, Hoenderop JG. Calcium and phosphate homeostasis: concerted interplay of new regulators. Ann Med 2008;40:82–91 - PubMed
-
- Hoenderop JG, Nilius B, Bindels RJ. Calcium absorption across epithelia. Physiol Rev 2005;85:373–422 - PubMed
-
- Ketteler M, Rothe H, Kruger T, Biggar PH, Schlieper G. Mechanisms and treatment of extraosseous calcification in chronic kidney disease. Nat Rev Nephrol 2011;7:509–16 - PubMed
-
- Kagawa T, Kozai M, Masuda M, Harada N, Nakahashi O, Tajiri M, Yoshikawa R, Nakao M, Takei Y, Iwano M, Takeda E, Taketani Y, Yamamoto H. Sterol regulatory element binding protein 1 trans-activates 25-hydroxy vitamin D3 24-hydroxylase gene expression in renal proximal tubular cells. Biochem Biophys Res Commun 2018;500:275–82 - PubMed
-
- Ghazarian JG, Yanda DM. Inhibition of 25-hydroxyvitamin D 1α-hydroxylase by renal mitochondrial protein kinase-catalyzed phosphorylation. Biochem Biophys Res Commun 1985;132:1095–102 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

