Siglec-F-expressing neutrophils are essential for creating a profibrotic microenvironment in renal fibrosis
- PMID: 35482420
- PMCID: PMC9197522
- DOI: 10.1172/JCI156876
Siglec-F-expressing neutrophils are essential for creating a profibrotic microenvironment in renal fibrosis
Abstract
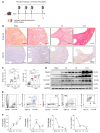
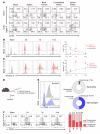
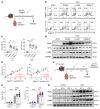
The roles of neutrophils in renal inflammation are currently unclear. On examining these cells in the unilateral ureteral obstruction murine model of chronic kidney disease, we found that the injured kidney bore a large and rapidly expanding population of neutrophils that expressed the eosinophil marker Siglec-F. We first verified that these cells were neutrophils. Siglec-F+ neutrophils were recently detected in several studies in other disease contexts. We then showed that a) these cells were derived from conventional neutrophils in the renal vasculature by TGF-β1 and GM-CSF; b) they differed from their parent cells by more frequent hypersegmentation, higher expression of profibrotic inflammatory cytokines, and notably, expression of collagen 1; and c) their depletion reduced collagen deposition and disease progression, but adoptive transfer increased renal fibrosis. These findings have thus unveiled a subtype of neutrophils that participate in renal fibrosis and a potentially new therapeutic target in chronic kidney disease.
Keywords: Chronic kidney disease; Fibrosis; Immunology; Nephrology; Neutrophils.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

