Effects of immune checkpoint inhibitor therapy resumption in patients with malignant tumors after moderate-to-severe immune-related adverse events
- PMID: 35482642
- PMCID: PMC9049539
- DOI: 10.1371/journal.pone.0267572
Effects of immune checkpoint inhibitor therapy resumption in patients with malignant tumors after moderate-to-severe immune-related adverse events
Abstract
Background and aims: Immune checkpoint inhibitors (ICIs) are used to treat several cancers, but they sometimes induce immune-related adverse events (irAEs). Patients with irAEs often have improved antitumor responses, but discontinuation of ICIs after irAEs is considered necessary. Resuming the use of ICIs after irAEs is preferable, but few studies have investigated the safety of ICI resumption after irAEs. Therefore, we evaluated the factors associated with the recurrence of irAEs after ICI resumption to investigate the safety of this approach.
Methods: In this observational study, we enrolled patients treated with ICIs from September 2014 to March 2020 at our institution. Patient characteristics, ICIs, grades of irAEs, ICI discontinuation or resumption rates, and recurrence rates of irAEs after ICI therapy were analysed.
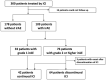
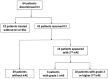
Results: Two-hundred eighty-seven patients were included in the present study, and 76 patients experienced grade 2 or higher irAEs. Forty-two patients underwent ICI resumption after recovering from irAEs, and 13 of them had a recurrence of irAEs. Among those 13 patients, six had a recurrence of the same irAE, and seven experienced other irAEs. Ten of the 13 patients had grade ≥2 irAEs, and none had fatal irAEs. In the grade 2 or higher irAE group, more patients had irAEs associated with multiple organs and of initial grade ≥2 than those in the grade 1 and no recurrent irAEs group.
Conclusions: Patients with initial multisystemic irAEs and irAEs of grade ≥2 were more likely to experience relapse or develop new grade ≥2 irAEs after ICI resumption.
Conflict of interest statement
Dr. Ishitsuka holds has received a research fund from ONO PHARMACEUTICAL CO., LTD. The remaining authors have no financial relationships to disclose that are relevant to this publication.
Figures
Similar articles
-
Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade ≥2 immune-related adverse events in patients with cancer.J Immunother Cancer. 2020 Dec;8(2):e001622. doi: 10.1136/jitc-2020-001622. J Immunother Cancer. 2020. PMID: 33428586 Free PMC article.
-
Patterns and outcomes of immune-related adverse events in solid tumor patients treated with immune checkpoint inhibitors in Thailand: a multicenter analysis.BMC Cancer. 2021 Nov 25;21(1):1275. doi: 10.1186/s12885-021-09003-z. BMC Cancer. 2021. PMID: 34823493 Free PMC article.
-
Real-World Clinical and Economic Outcomes in Selected Immune-Related Adverse Events Among Patients with Cancer Receiving Immune Checkpoint Inhibitors.Oncologist. 2021 Nov;26(11):e2002-e2012. doi: 10.1002/onco.13918. Epub 2021 Aug 24. Oncologist. 2021. PMID: 34327774 Free PMC article.
-
Safety and Efficacy of the Rechallenge of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients With Cancer: A Systemic Review and Meta-Analysis.Front Immunol. 2021 Sep 27;12:730320. doi: 10.3389/fimmu.2021.730320. eCollection 2021. Front Immunol. 2021. PMID: 34646270 Free PMC article.
-
Safety of Immune Checkpoint Inhibitor Resumption after Interruption for Immune-Related Adverse Events, a Narrative Review.Cancers (Basel). 2022 Feb 14;14(4):955. doi: 10.3390/cancers14040955. Cancers (Basel). 2022. PMID: 35205703 Free PMC article. Review.
Cited by
-
Endocrine Side Effects in Patients Treated with Immune Checkpoint Inhibitors: A Narrative Review.J Clin Med. 2023 Aug 7;12(15):5161. doi: 10.3390/jcm12155161. J Clin Med. 2023. PMID: 37568563 Free PMC article. Review.
-
Immune Checkpoint Inhibitor-Associated Myocarditis: A Literature Review.Cureus. 2024 Jan 25;16(1):e52952. doi: 10.7759/cureus.52952. eCollection 2024 Jan. Cureus. 2024. PMID: 38406102 Free PMC article. Review.
References
-
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al.. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37 (7): 537–546. doi: 10.1200/JCO.18.00149 - DOI - PubMed
-
- Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthélémy P, et al.. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 Trial. ESMO Open. 2020;5 (6): e001079. doi: 10.1136/esmoopen-2020-001079 - DOI - PMC - PubMed
-
- Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al.. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18 (9): 1182–1191. doi: 10.1016/S1470-2045(17)30422-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical