New insights and advances on pyomelanin production: from microbial synthesis to applications
- PMID: 35482661
- PMCID: PMC9338888
- DOI: 10.1093/jimb/kuac013
New insights and advances on pyomelanin production: from microbial synthesis to applications
Abstract
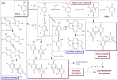
Pyomelanin is a brown-black phenolic polymer and results from the oxidation of homogentisic acid (HGA) in the L-tyrosine pathway. As part of the research for natural and active ingredients issued from realistic bioprocesses, this work re-evaluates the HGA pigment and makes an updated inventory of its syntheses, microbial pathways, and properties, with tracks and recent advances for its large-scale production. The mechanism of the HGA polymerization is also well documented. In alkaptonuria, pyomelanin formation leads to connective tissue damage and arthritis, most probably due to the ROS issued from HGA oxidation. While UV radiation on human melanin may generate degradation products, pyomelanin is not photodegradable, is hyperthermostable, and has other properties better than L-Dopa melanin. This review aims to raise awareness about the potential of this pigment for various applications, not only for skin coloring and protection but also for other cells, materials, and as a promising (semi)conductor for bioelectronics and energy.
Keywords: Applications; Hydroxylase; Laccases; Polymerization; Pyomelanin.
© The Author(s) 2022. Published by Oxford University Press on behalf of Society of Industrial Microbiology and Biotechnology.
Figures





Similar articles
-
Identification and molecular characterization of the homogentisate pathway responsible for pyomelanin production, the major melanin constituents in Aeromonas media WS.PLoS One. 2015 Mar 20;10(3):e0120923. doi: 10.1371/journal.pone.0120923. eCollection 2015. PLoS One. 2015. PMID: 25793756 Free PMC article.
-
Production of pyomelanin, a second type of melanin, via the tyrosine degradation pathway in Aspergillus fumigatus.Appl Environ Microbiol. 2009 Jan;75(2):493-503. doi: 10.1128/AEM.02077-08. Epub 2008 Nov 21. Appl Environ Microbiol. 2009. PMID: 19028908 Free PMC article.
-
Homogentisic acid is the primary precursor of melanin synthesis in Vibrio cholerae, a Hyphomonas strain, and Shewanella colwelliana.Appl Environ Microbiol. 1995 Apr;61(4):1620-2. doi: 10.1128/aem.61.4.1620-1622.1995. Appl Environ Microbiol. 1995. PMID: 7747976 Free PMC article.
-
Alkaptonuria: a very rare metabolic disorder.Indian J Biochem Biophys. 2013 Oct;50(5):339-44. Indian J Biochem Biophys. 2013. PMID: 24772955 Review.
-
Ochronotic pigmentation is caused by homogentisic acid and is the key event in alkaptonuria leading to the destructive consequences of the disease-A review.J Inherit Metab Dis. 2019 Sep;42(5):776-792. doi: 10.1002/jimd.12152. Epub 2019 Aug 5. J Inherit Metab Dis. 2019. PMID: 31282009 Review.
Cited by
-
Genome mining the black-yeast Aureobasidium pullulans NRRL 62031 for biotechnological traits.BMC Genomics. 2025 Mar 13;26(1):244. doi: 10.1186/s12864-025-11395-2. BMC Genomics. 2025. PMID: 40082747 Free PMC article.
-
Exploring the Osteoinductive Potential of Bacterial Pyomelanin Derived from Pseudomonas aeruginosa in a Human Osteoblast Model.Int J Mol Sci. 2024 Dec 14;25(24):13406. doi: 10.3390/ijms252413406. Int J Mol Sci. 2024. PMID: 39769171 Free PMC article.
-
Lomentospora prolificans synthesizes several types of melanin.mSphere. 2025 Apr 29;10(4):e0096324. doi: 10.1128/msphere.00963-24. Epub 2025 Apr 2. mSphere. 2025. PMID: 40172218 Free PMC article.
-
Heterologous production and characterization of a pyomelanin of Antarctic Pseudomonas sp. ANT_H4: a metabolite protecting against UV and free radicals, interacting with iron from minerals and exhibiting priming properties toward plant hairy roots.Microb Cell Fact. 2022 Dec 16;21(1):261. doi: 10.1186/s12934-022-01990-3. Microb Cell Fact. 2022. PMID: 36527127 Free PMC article.
-
Can Pyomelanin Produced by Pseudomonas aeruginosa Promote the Regeneration of Gastric Epithelial Cells and Enhance Helicobacter pylori Phagocytosis?Int J Mol Sci. 2023 Sep 10;24(18):13911. doi: 10.3390/ijms241813911. Int J Mol Sci. 2023. PMID: 37762213 Free PMC article.
References
-
- Abdul-Hussien Z.R., Atia S.S. (2017). Antimicrobial effect of pyomelanin extracted from Pseudomonas aeruginosa. International Journal Development Research, 7, 8692. https://www.journalijdr.com/antimicrobial-effect-pyomelanin-extracted-ps...
-
- Almeida-Paes R., Almeida-Silva F., Pinto G.C.M., Almeida M.A., Muniz M.M., Pizzini C.V., Gerfen G.J., Nosanchuk J.D., Zancopé-Oliveira R.M. (2018). L-tyrosine induces the production of a pyomelanin-like pigment by the parasitic yeast-form of Histoplasma capsulatum. Medical Mycology, 56(4), 506–509. 10.1093/mmy/myx068 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

